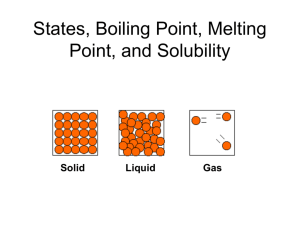
chemical and physical properties
advertisement

Page 64 Nov. 5, 2012 Focus: Chemical and Physical Properties / Changes Objective: pre-test, define physical and chemical property, physical and chemical change HW: article, data – analysis - graph due Warm-Up: • Is Humpty Dumpty falling off the wall and cracking on the ground a physical or chemical change? • Explain your answer. Pre-Assessment / Formative • • • • Name November 5, 2012 Block Title: Formative of Objectives 6.P.2.3, 6.P.3.1, 6.P.3.3 No more than 25 minutes Homework pg. 63 • Science Fair Project • Article and questions • Bring back card for My Energy Kit Physical versus Chemical Properties Reviewing MATTER • Matter: anything that has mass and takes up space – Mass – the amount of matter in something – Volume – the amount of space something occupies • Which of the following is matter? – A car? – A box? – You? What is a property? • Property: a characteristic of a substance that can be observed http://www.gpb.org/chemistryphysics/chemistry/201 States, Boiling Point, Melting Point, and Solubility Solid Liquid Gas Defining States of Matter • States of matter are NOT defined by what they are made of. – Example: solids can be elements (gold), compounds (Salt = NaCl), or mixtures (butter) Element (Au) Compound (NaCl) Mixture (Milk, Salt, etc) Defining States of Matter • States of matter are defined by whether they hold SHAPE and VOLUME Element (Au) Compound (NaCl) Mixture (Milk, Salt, etc) ALL KEEP THE SAME SHAPE AND VOLUME = Solids Defining States of Matter • Solids – have a definite SHAPE and VOLUME. Element (Au) Compound (NaCl) Mixture (Milk, Salt, etc) ALL KEEP THE SAME SHAPE AND VOLUME Particle View of a Solid • Particles in a solid are PACKED CLOSELY together and they are in a FIXED POSITION. Particles vibrate in place Liquids • Liquids – has definite VOLUME but no defined SHAPE 100 ml Particle View of a Liquid • Packed CLOSELY (like a solid), but move FREELY around each other (must stay in contact). Gases • Gases - do NOT have definite SHAPE or VOLUME. Bromine gas fills up the entire volume of the container Particle view of a Gas • Particles can MOVE FREELY and will either fill up or squeeze into available space. Changes in States of Matter • Thermal Energy – heat energy. • More thermal energy = More particle movement Changing States Increase Thermal Energy (Heat up) Solid Liquid Decrease Thermal Energy (Cool off) Gas Melting point • Melting - change from solid to liquid • Melting point - SPECIFIC temperature when melting occurs. • Each pure substance has a SPECIFIC melting point. – – – – – Examples: M.P. of Water = 0°C (32°F) M.P. of Nitrogen = -209.9 °C (-345.81998 °F) M.P. of Silver = 961.93 °C (1763.474 °F) M.P. of Carbon = 3500.0 °C (6332.0 °F) Melting Point • Particles of a solid vibrate so fast that they break free from their fixed positions. Increasing Thermal Energy Solid Liquid Melting point Vaporization • Vaporization – change from liquid to gas • Vaporization happens when particles in a liquid gain enough energy to form a gas. Increasing Thermal Energy Liquid Boiling point Gas Two Kinds of Vaporization • Evaporation – vaporization that takes place only on the surface of the liquid • Boiling – when a liquid changes to a gas BELOW its surface as well as above. Boiling Point • Boiling Point – temperature at which a liquid boils • Each pure substance has a SPECIFIC boiling point. – Examples: – B.P. of Water = 100°C (212°F) – B.P. of Nitrogen = -195.79 °C (-320.42 °F) – B.P. of Silver = 2162 °C (3924 °F) – B.P. of Carbon = 4027 °C (7281 °F) Boiling Point and Melting Point WATER – H20 200 Boiling point Temperature 150 100 Melting point 50 0 -50 -100 -150 time Solubility • Maximum amount of a substance that can be dissolved in a liquid (at a specific temperature). Salt (NaCl) Water (H20) at 20°C Solubility • Solute – substance being dissolved • Solvent – liquid substance that solute is dissolved into Salt (NaCl) Water (H20) at 20°C Solubility • Solute – ?????? Salt • Solvent – ????? Water Salt (NaCl) Water (H20) at 20°C Solubility can change • Increased Temp = Increased Solubility • Different substances have different solubility curves Solubility of Unknow n Substance at different tem peratures substance/ml of H20 mg of unknown 3 2.5 2 1.5 1 0.5 0 1 3.8 6.6 9.4 12.2 15 17.8 tem perature (degrees Celcius) 20.6 23.4 26.2 Physical Property Physical property: a property that can be observed without changing the identity of the substance. Examples: • luster • melting point • malleability: the ability to be hammered into a thin sheet • boiling point • ductility: the ability to be stretched into a wire • solubility • density • specific heat Special Physical Properties • Melting point: the temperature at which a substance changes from a solid to a liquid at a given pressure water = 0oC • Boiling point: the temperature at which a substance changes from a liquid to a gas at a given pressure water = 100oC Chemical Properties • Chemical property: a property that can only be observed by changing the identity of the substance Examples: •flammability •ability to rust •reactivity with vinegar Chemical Properties & Physical and Chemical Changes Physical changes are those changes that do not result in the production of a new substance. If you melt a block of ice, you still have H2O at the end of the change. If you break a bottle, you still have glass. Painting your nails will not stop them from being fingernails. Some common examples of physical changes are: melting, freezing, condensing, breaking, crushing, cutting, and bending. Some, but not all physical changes can be reversed. You could refreeze the water into ice, but you cannot put your hair back together if you don’t like your haircut! Special types of physical changes where any object changes state, such as when water freezes or evaporates, are sometimes called change of state operations. CHEMICAL PROPERTIES Chemical properties can ONLY be observed AS the substances are changing into different substances. Chemical changes, or chemical reactions, are changes that result in the production of another substance. FLAMMABILITY: A material’s ability to BURN in the presence of OXYGEN REACTIVITY: How readily (easily) a substance combines chemically with other substances. When you burn a log in a fireplace, you are carrying out a chemical reaction that releases carbon. When you light your Bunsen burner in lab, you are carrying out a chemical reaction that produces water and carbon dioxide. Common examples of chemical changes that you may be somewhat familiar with are; digestion, respiration, photosynthesis, burning, and decomposition. Physical or Chemical Change? • Painting Wood • PHYSICAL Physical or Chemical Change? • Burning Paper • CHEMICAL Physical or Chemical Change? • Digestion of food • CHEMICAL Physical or Chemical Change? • Sugar dissolving in water • PHYSICAL Physical or Chemical Change? • Iron turning red when heated • PHYSICAL Physical or Chemical Change? • Evaporation • PHYSICAL Physical or Chemical Change? • A pond freezing in winter • PHYSICAL Physical or Chemical Change? • Melting ice • PHYSICAL Physical or Chemical Change? • Cutting wire • PHYSICAL Physical or Chemical Change? • Painting fingernails • PHYSICAL Physical or Chemical Change? • Cutting fabric • PHYSICAL Physical or Chemical Change? • Baking muffins • CHEMICAL Physical or Chemical Change? • Shattering glass • PHYSICAL Physical or Chemical Change? • Decomposition of old leaves • CHEMICAL Physical or Chemical Change? • Wrinkling a shirt • PHYSICAL Physical or Chemical Change? • An old nail rusting • CHEMICAL Density • Density is the amount of mass per unit of volume. • Density can be used to identify a substance. • The density of water is 1.0g/mL Density Calculations • Calculations: D = m/V = g/mL = g/cm3 • Ex: A cube has a mass of 2.8 g and occupies a volume of 3.67 ml. Would this object float or sink in water? Mass = 2.8 g Volume = 3.67 mL D = 2.8g/3.67 mL= 0.76 g/mL – This object would float in water because its density is less than water (1.0 g/mL). More Density Calculations • Ex: A liquid has a mass of 25.6 g and a volume of 31.6 mL. Use the table below to identify the substance. Substance Density (g/mL) D = 25.6 g/31.6 mL Mercury 13.6 D= 0.81 g/mL Water 1.00 Ethanol 0.81 M=25.6 g V=31.6 mL The substance is ethanol. • Bingo • clickr