MS1005N General Biochemistry
Dr Sundus Tewfik s.tewfik@londonmet.ac.uk
Learning outcomes:
Define “lipid” and classify lipids by their chemistry;
Draw saturated & unsaturated fatty acids;
Illustrate a steroid, a TG and a PL;
1. More commonly known as fats or oils
2. Includes many compounds which are poorly
soluble in water
3. Many are amphiphiles (meaning that they
are soluble in both polar and non-polar solvents)
4.
some of these are weak amphiphiles eg
triglycerides and poorly soluble in water while
others are strong amphiphiles eg phospholipids
5.
solvents capable of dissolving lipids are
called fat solvents and include benzene, diethyl
ether, chloroform. A common solvent mixture is
chloroform:methanol.
diethyl ether
chloroform
benzene
methanol
6.
The following classification is one which is often
used. There are 5 classes recognised;
I
fatty acids - these are long chain carboxylic acids
RCOOH
II
acylglycerols, glycerides or neutral fats - these
are neutral esters of glycerol and fatty acids
Esters; A carboxylic acid
contains the -COOH group, and
in an ester the hydrogen in this
group is replaced by a
hydrocarbon group of some kind.
III
phosphoacylglycerols or phosphoglycerides - ionic esters
of glycerol, fatty acids and phosphate
IV
lipids (apart from fatty acids) not containing glycerol includes waxes, polyprenyls, terpenes and steroids
V
lipids combined with other biomolecules - such as
proteins or carbohydrates
Lipoproteins
lipopolysaccharides
7.
FATTY ACIDS
it is necessary to distinguish between free fatty acids
(FFAs) which are not combined with other structures and
those fatty acids (FAs) which are found as residues as
part of other lipids such as glycerides and
phosphoglycerides
8.
There are only small amounts of FFAs found in
nature the remaining FAs are combined into other
structures.
9.
Fatty acids
have an ionisable carboxyl group and a nonpolar
unbranched hydrocarbon chain
RCOOH
RCOO- + H
•usually possess an even number of carbon atoms
(acetyle CoA)
•can be saturated (no carbon-carbon double bonds)
or unsaturated (possess some carbon-carbon
double bonds )
-C=C•nomenclature (systematic naming) is very important since it aids
understanding of structure
6
5
4
3
2
1
number of C atom
CH3CH2CH2CH2CH2COOH
•the systematic name of the above
compound is based on the number of
carbon atoms and is related to alkane
structure and the fact that it is a carboxylic acid
alkane
•(Can you suggest its name?)
•it is n-hexanoic acid and it has a common name,
•caproic acid
10. In animal fats the most abundant saturated
fatty acids are palmitic acid (C16 ie hexadecanoic
acid) and stearic acid (C18 ie octadecanoic acid)
Palmitic acid - saturated
stearic acid-Saturated
11. C12, C14 and C20-C28 FAs do occur in animal
fats but in much smaller quantities. FAs below C10
occur very rarely in animal fats
12. Unsaturated FAs contain carbon:carbon double
bondsand their names end in enoic as follows
number of double bonds
suffix (name ending)
1
2
3
4
5
-enoic
-dienoic
-trienoic
-tetraenoic
-pentaenoic
13.
How would you name the following unsaturated FA?
(Hint - the numbering of the double bond position is taken from the
lowest numbered carbon atom involved in the double bond)
number of C atom
14.
16 15-11 10 9 8-2 1
CH3[CH2]5CH=CH[CH2]7COOH
15.
9- hexadecenoic acid (common name - palmitoleic acid)
16.
even this apparently complex systematic name does not
define it completely since unsaturated FAs can exist in cis or trans
forms ( hint - revise geometric isomerism)
Cis Configuration
Trans Configuration
Since the trans acids are straighter than their bent cis isomers, they can
pack together easier and so have a higher melting point. By selecting a
particular hydrogenation catalyst, temperature, stirring speed and pressure,
manufacturers can control the precise composition of the margarine to
create a particular 'mouth feel', melting range and stability.
unlike the cis acids, which occur
naturally, the trans acids are artificial
and so cannot be metabolised in the
human body as efficiently as their
isomers. Over the past 3 decades TFAs
have been linked to cancer, diabetes,
heart disease, low birth rate and
obesity.
9 octadecenoic acid
17. In polyunsaturated FAs (PUFAs) the double
bonds are usually separated as follows:
-[CH=CH-CH2-CH=CH]-
divinyl methane structure
but
also the following may be found:
-[CH=CH-CH=CH]- conjugated diene structure.
18.
In animal tissues the most commonly found
PUFAs are
linoleic acid
18:2
linolenic acid
18:3
arachidonic acid 20:4
19.
Note also that the position of the double bonds can be
given in shorthand form, thus oleic acid is 18:1 Δ9
20.
The last carbon atom is denoted as ω thus the position of
the last (and only) double bond in oleic acid is ω -9
21.
What is the ω position of linolenic acid 18:3 Δ9,12,15 ?
A. ω-3
22.
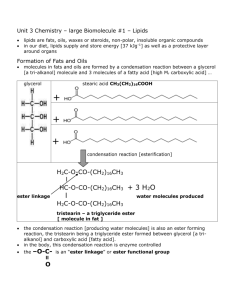
TRIACYLGLYCEROLS (OR TRIGLYCERIDES)
•commonly occur as storage fats in, for
example, adipose tissue; they provide a
reservoir of energy
•they are neutral fats
•they comprise 3 fatty acids combined with
glycerol
•note that mono and diglycerides also exist as
storage fats
CH2OH
CHOH
CH2OH
glyserol
+
3 HOOC[CH2]16CH3
stearic acid
esterification
CH2O.OC[CH2]16CH3
CHO.OC[CH2]16CH3 +
CH2O.OC[CH2]16CH3
tristearin
3H2O
the reverse of this process is a form of hydrolysis called
lipolysis. This may be carried by enzymes called lipases
yielding FAs and glycerol. In the case of FAs a process
called β oxidation followed by the TCA cycle and
oxidative phosphorylation provides a large yield of ATP
Formation of a Triglyceride via Dehydration Synthesis
Condensation Reactions (Building of larger molecules) add energy to organic
molecules.
Hydrolysis Reactions (those that tear apart molecules) release energy from the
molecules.
http://www.lipidsonline.org/
23.
PHOSPHOACYLGLYCEROLS (OR
PHOSPHOGLYCERIDES, GLYCEROPHOSPHOLIPIDS)
these are strong amphiphiles
they are found in biological membranes
CH2OH
HOCH
O
CH2 O - P - O
O-
+
glycerol 3 phosphate
2 FAs
usually saturated
CH2O.OC[CH2]nCH3
CH3[CH2]nCO.OCH
may be unsaturated
What has this got to do
with reindeer?
O
CH2 O - P - O
O-
+ 2H2O
phosphatidic acid
phosphatidic acid is both an example of a phosphoacylglycerol
and a precursor of phosphoacylglycerols.
24.
LIPIDS (APART FROM FAs) NOT CONTAINING
GLYCEROL
these include structures based on isoprene units
head
tail
isoprene
lipids which contain the isoprene arrangement are called
polyprenyls (terpenes if they are of plant origin)
polyprenyls
examples include fat soluble vitamins such as vitamin K and vitamin E (α-tocopherol)
Vitamin K
Vitamin E (α- tocopherol)
other fat-soluble vitamins are A (trans-retinol) and D (cholecalciferol)
26.
STEROIDS
these have the characteristic steroid ring structure :-
they occur widely in all types of living organisms except
prokaryotes
versatile group of compounds often found as hormones
27.
The most abundant steroid in the human body is
cholesterol (~ 240g per body) which is essential for proper
membrane function
28.
Cholesterol is often viewed as a ‘bad’ molecule despite
having a varied essential and normal roles in the human body.
29.
This image stems from a possible correlation between
blood cholesterol levels and risk of heart disease.
30.
Some cholesterol is converted to other compounds eg
bile salts, sex hormones, adrenal hormones and vitamin D
(cholecalciferol)
31.
The corticosteroids are products of the adrenal cortex
which help regulate the metabolism of electrolytes, proteins and
carbohydrates
32.
Human sex hormones (and their derivatives) are also
steroids - these include testosterone (male sex hormones),
and progesterone and œstradiol (female sex hormones)
Commercially, derivatives of these female hormones
are used in birth control pills and therapeutic agents
such as those designed to treat post-menopausal
symptoms. The male hormone and derivatives can be
abused as anabolic steroids where they increase muscle
production (but also increase aggressiveness, liver
damage and heart disease)
33.
Bile includes steroids such as taurocholate. This is a
bile salt which is an amphipathic steroid (how might this
occur?)
A.
it has a polar and and a non-polar part to its structure
hydrophilic
34.
These bile salts aid digestion in the small intestine by
helping nonpolar lipids mix with the more polar digestive
enzymes
Study questions
1. A lipid is a polymer made up of which kind of monomers?
a) amino acids
c) nucleotides
b) alternating sugar and phosphate groups
d) fatty acids and glycerol
2. A dehydration synthesis reaction is also known as
a) a condensation reaction
b) a hydrolysis reaction
c) an isomeric reaction
d) an energy releasing reaction
e) monomer formation
3. Which of the following lipids form a bilayer between two watery regions,
such as the plasma membrane of a cell?
a) steroids b) neutral fats c) waxes d) phospholipids
4. Which of the following is Not a lipid?
a) steroids b) fats c) phospholipids d) glycogen
5. Which of the following lipids is saturated?
e) cholesterol