Kraft Pulping Kinetics
advertisement
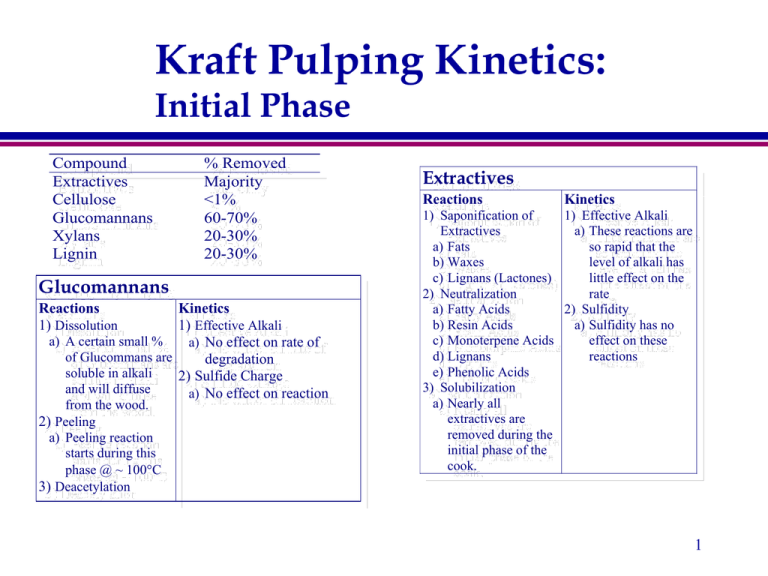
Kraft Pulping Kinetics: Initial Phase Compound Extractives Cellulose Glucomannans Xylans Lignin % Removed Majority <1% 60-70% 20-30% 20-30% Glucomannans Reactions 1) Dissolution Kinetics 1) Effective Alkali a) A certain small % a) No effect on rate of of Glucommans are degradation soluble in alkali 2) Sulfide Charge and will diffuse a) No effect on reaction from the wood. 2) Peeling a) Peeling reaction starts during this phase @ ~ 100C 3) Deacetylation Extractives Reactions Kinetics 1) Saponification of 1) Effective Alkali Extractives a) These reactions are a) Fats so rapid that the b) Waxes level of alkali has c) Lignans (Lactones) little effect on the 2) Neutralization rate a) Fatty Acids 2) Sulfidity b) Resin Acids a) Sulfidity has no c) Monoterpene Acids effect on these d) Lignans reactions e) Phenolic Acids 3) Solubilization a) Nearly all extractives are removed during the initial phase of the cook. 1 Kraft Pulping Kinetics: Initial Phase Xylans Lignin Reactions Kinetics 1) Cleavage of -O-4 1) Effective Alkali a) No affect on linkages rate during this a) Units with free phase phenolic hydroxyl b) Rate diffusion b) Very fast limited in this 2) Cleavage of -O-4 phase linkages 2) Sulfidity a) Units with free a) Sulfidity has no phenolic hydroxyl effect during or carbonyl. this phase b) Rapid reaction 3) Solubilization a) Small amount of lignin alkali soluble 4) Condensation a) Limited Reactions Kinetics 1) Dissolution 1) Effective Alkali a) Major reaction of a) Higher EA increases the Xylans during kraft rate of dissolution pulping 2) Sulfide Charge 2) Peeling a) No effect on reaction a) Very minor reaction starts @ ~ 100C 3) Stopping a) See 2a 4) Deacetylation Cellulose Reactions Kinetics 1) Peeling 1) Effective Alkali a) Loss of cellulose begins a) ? 2) Sulfide Charge @ ~ 120C - 130 C 2) Minor reaction as only 10% a) No effect on reaction of all cellulose lost during cook. 2 Kraft Pulping Kinetics: Bulk Phase Compound Extractives Cellulose Glucomannans Xylans Lignin % Removed (total) Majority <10% 60-70% 30-50% 80-85% Cellulose Reactions Kinetics 1) Peeling 1) Effective Alkali a) Loss of cellulose a) ? accelerates at temperature 2) Sulfide Charge due to glycosidic cleavage a) No effect on reaction 2) Stopping a) Stopping reactions slow loss of cellulose 3) Glycosidic Cleavage a) See 1a b) Starts to reduce cellulose viscosity Lignin Reactions Kinetics 1) Cleavage of non 1) Effective Alkali a) Higher EA = phenolic -O-4 faster rate of followed by: degradation a) Cleavage of -O-4 2) Sulfide Charge linkages in units a) Higher sulfide with free phenolic charge increases hydroxyl the rate of lignin b) Cleavage of -O-4 removal. linkages in units b) Higher lignin with free phenolic removal rate hydroxyl Rapid results in a reaction lower lig/carb at c) Condensation end of cook d) Cleavage of C-C bonds (double) 3 Kraft Pulping Kinetics: Bulk Phase Xylans Reactions Glucomannans Kinetics 1) Dissolution 1) Effective Alkali a) Major reaction of a) Higher EA increases the Xylans during this rate of dissolution phase 2) Sulfide Charge 2) Peeling a) No effect on reaction a) Minor reaction 3) Glycosidic Cleavage a) Will increase rate of peeling reaction 4) Precipitation on fibers a) Starts as alkali consummed Reactions 1) Peeling Kinetics 1) Effective Alkali a) Peeling is b) No effect on rate of extensive until degradation about 70% of xylan 2) Sulfide Charge is lost and then a) No effect on reaction almost stops 2) Gylcosidic cleavage a) Increases the rate of peeling 3) Stopping a) Stops losses due to peeling 4 Kraft Pulping Kinetics: Residual Phase Compound Extractives Cellulose Glucomannans Xylans Lignin % Removed (total) Majority ~10% 70-80% 40-50%% 85-95% Cellulose Reactions 1) Glycosidic Cleavage Kinetics 1) Effective Alkali a) Major reaction resulting in a) ? 2) Sulfide Charge lower molecular weight a) No effect on reaction and strength loss 2) Peeling/stopping a) Minor reactions Lignin Reactions Kinetics 1) Majority of ether 1) Effective Alkali linkages that will a) The rate slows cleave are gone: rapidly: 2) Cleavage of C-C b) amount of lignin bonds (double) remaining a 3) Condensation function of the a) Increase the Mw EA and sulfide and lower reactivity charge during of remaining lignin the cook 5 Kraft Pulping Kinetics: Residual Phase Xylans Reactions 1) Peeling/stopping/ a) negligible 2) Precipitation on fibers a) Significant amount deposited as alkali continues to be consummed Kinetics 1) Effective Alkali a) Lower levels of alkali at end of the cook casuse deposition of xylans 2) Sulfide Charge a) No effect on reaction Glucomannans Reactions 1) Peeling Kinetics 1) Effective Alkali a) Limited b) No effect on rate of 2) Gylcosidic cleavage degradation a) Limited 2) Sulfide Charge 3) Stopping a) No effect on reaction a) Limited 6 Kraft Pulping Kinetics: In “Nutshell” Phase Lignin Carbohydrate Alkali Initial Rapid delignification Indep. of [OH-] and [HS-] Rapid carbohydrate Rapid alkali degradation consumption Some [OH-] dependence Indep.of [HS-] Bulk Moderate delignification rate Dep. on [OH-] and [HS-] Moderate carbohydrate degradation Moderate consumption of alkali 7 Kraft Pulping Kinetics: In “Nutshell” Phase Lignin Carbohydrate Alkali Residual Very slow delignification Some dep. on [OH-] and ? [HS-] Substantial Some alkali carbohydrate consumption degradation – especially viscosity Re-deposition of Xylans 8