Properties of acids and bases
advertisement

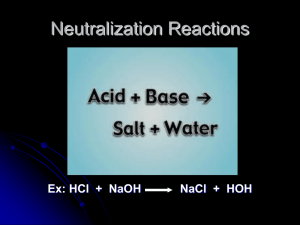
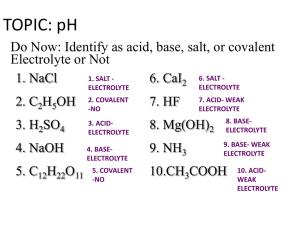
Properties of acids and bases Sour/tart taste Conduct electricity Litmus turns red Release H+ into water pH < 7 Neutralize a base Usually start with H Most food items Slippery to the touch Bitter taste Conduct electricity Litmus turns blue Release OH- into water Neutralize an acid pH > 7 Most cleaning items An acid is a compound that gives H+ (or H3O+) in water. Acids generally begin with H. HCl + H2O H3O+1 + Cl-1 HCl H+1 + Cl–1 A base is a compound that gives OH- in water. Bases usually end in –OH. NaOH Na+1 + OH-1 An indicator is a colored substance that can exist in either an acid or base solution. Two common indicators: Litmus paper: red blue 6.8 7.3 Phenolphthalein: clear pink 8.3 8.4 Monoprotic: acids or bases that give off one H+1(acid) or OH-1(base) HCl H+1 + Cl-1 NaOH Na+1 + OH-1 Diprotic: acids or bases that give off two H+1 (acid) or two OH-1(base) H2SO4 2 H+1 + SO4-2 Ca(OH)2 Ca+2 + 2OH-1 Triprotic: acids or bases that give off three H+1(acid) or three OH-1(base) H3PO4 3H+1 + PO4-3 Al(OH)3 Al+3 + 3 OH-1 Ions – charged particles Naming acids Common acids normally begin with H. When you see a compound that begins with H, it should be named as an acid. The name of the acid depends on the negative ion: •If the name of the anion ends in –ide Hydro (anion without –ide) –ic acid HCl – hydrochloric acid HBr – hydrobromic acid H2S – hydrosulfuric acid •If the anion ends in –ate, everything is the same except do not add the prefix hydro(anion name without –ate) –ic acid HNO3 – nitric acid HClO3 – chloric acid H2SO4 – sulfuric acid Common acids and bases HCl HNO3 H2SO4 HC2H3O2 HBr H2CO3 H3PO4 NaOH KOH Ca(OH)2 Mg(OH)2 Ba(OH)2 Bronsted Acid-Bases Acid – a substance that gives up H+ Conjugate Base – what is left after the acid gives up H+ Base – a substance that gains H+ Conjugate Acid – what is left after the base gains H+ Plain acids and bases are on the left side of a reaction, the conjugate acid and base are on the right side of a reaction. C2H3O2-1 + NH4+1 HC2H3O2 + NH3 H2O + H3PO4 H3O+1 + H2PO4-1 H2O + NH3 OH-1 + NH4+1 [ ] = concentration (unit of molarity) In pure water at 25oC [H+1] = 1.00 E-7 M [OH-1] = 1.00 E-7 M [H+1]x[OH-1] = 1.00E-7 x 1.00E -7 = 1.00E-14 One way of expressing the [H+1] or the [OH-1] is the pH. pH = -log[H+1] pOH = -log[OH-1] Important Formulas: pH + pOH = 14.00 [H+1] x [OH-1] = 1.00 E-14 pH = -log[H+1] pOH = -log[OH-1] pH Scale 06.99 acid 7.00 neutral 7.01 14.00 base To calculate the pH (or the pOH) 1. (-) 2. log 3. [H+1] (or [OH-1] To calculate [H+1] (or [OH-1]) 1. 10x (2nd log) 2. (-) 3. pH (or pOH) Neutralization Reactions An acid will react with a base and neutralize each other. The result when they are mixed is ALWAYS salt and water. Acid + Base Salt + Water HA + BOH BA + HOH HCl + NaOH NaCl + HOH To predict the products of neutralization reactions: 1. Recognize that it is a double replacement reaction 2.Pair up the new products: one will always be HOH (or water, H2O) 3.You can get the correct charges from H+1 or OH-1 4.Get the correct formulas by crossing the charges 5.Balance the reaction H2SO4 + Al(OH)3 • H2SO4 + Al(OH)3 HOH + AlSO4 (pair up new products) • H2SO4 + Al(OH)3 HOH + Al2(SO4)3 (get the correct formulas) • 3H2SO4 + 2Al(OH)3 6HOH + Al2(SO4)3 (balance the reaction) Titration – a solution of a known concentration is reacted with a known volume of a solution of unknown concentration. At the endpoint, an indicator will change colors. At that point, the [H+1] = [OH-1]. From this information, the unknown concentration can be determined. 1. Write a balanced reaction 2. Label all numbers 3. Use unit analysis 4. Start with the volume of the compound that you know both volume and concentration 5. vol moles A moles B conc B 27.5 milliliters of H2SO4 is exactly neutralized by 39.3 milliliters of 0.437 M NaOH. What is the concentration of the acid? H2SO4 + 2 NaOH Na2SO4 + 2 HOH Acid: 27.5 ml Base: 39.3 ml 0.0393 L NaOH 0.437 M 0.437 mol NaOH 1 mol H2SO4 1 L NaOH 2 mol NaOH 1 = 0.0275 L H2SO4 0.312M H2SO4 There is an alternate way to do titration calculations that does not involve writing balanced reactions or unit analysis: PLHABTSM® M1V1P1 = M2V2P2 M1 = conc of acid M2 = conc of base V1 = volume of acid V2 = volume of base P1 = protacticity P2 = protacticity Protacticity P1 = # of H+1 per molecules P2 = # of OH-1 per molecules 1. Read the problem 2. Label all of the numbers 3. Identify the unknown 4. Use M1V1P1 = M2V2P2 5. Solve for the unknown 27.5 milliliters of H2SO4 is exactly neutralized by 39.3 milliliters of 0.437 M NaOH. What is the concentration of the acid? M1 = M2 = V1 = V2 = P1 = P2 =






![pH = - log [H + ]](http://s2.studylib.net/store/data/005622524_1-002df1ea50d2a849b15deb604928664e-300x300.png)