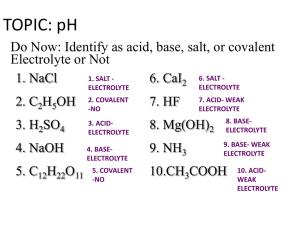
acids and bases 2. key
advertisement

Name: _________________________________________ Date:____________________________ Optional!!! Homework 11 – do not turn in!!! 1.) Given the following [H+1] concentrations, calculate pH, pOH, and [OH-1]. Are the solutions acidic or basic? a. [H+1] = 2.00 x 10-3 M pH = -log(H+1) pH = 2.70 ACIDIC pH = -log(2.00 x 10-3) = 2.70 pOH = 11.30 14.00 = pH + pOH 14.00 = 2.70 + pOH [OH-1] = 5.01 x 10-12 M 14.00 – 2.70 = pOH = 11.30 OR!!! pOH = -log[OH-1] 11.30 = -log[OH-1] 1.00 x 10-14 = [H+1][OH-1] [OH-1] = 1.00 x 10-14/2.00 x 10-3 = 5.00 x 10-12 M OH-1 10-11.30 = 5.01 x 10-12 M OH-1 pOH = -log[OH-1] = -log(5.00 x 10-12) = 11.30 b. [H+1] = 7.67 x 10-3 M pH = -log (7.67 x 10-3) = 2.11 pH = 2.11 ACIDIC 14.00 = pH + pOH 14.00 = 2.11 + pOH pOH = 11.88 pOH = 11.88 pOH = -log[OH-1] 11.88 = -log [OH-1] OH-1 = 10-11.88 = 1.32 x 10-12 M [OH-1] = 1.32 x 10-12 M 2.) Given the following pOH values, calculate your concentration of [H+1], [OH-1], and pH (don’t forget units!!) pH = 12.45 a. pOH = 1.55 [H+1] = 3.55 x 10-13 M 14.00 = pH + pOH 14.00 = pH + 1.55 [OH-1] = 2.82 x 10-2 M pH = 12.45 pH = -log[H+1] pOH= -log[OH-1] 10-12.45 = 3.55 x 10-13 M H+1 10-1.55 = 2.82 x 10-2 M OH-1 _______________________________________________________________________________________ b. pOH = 4.25 pH = 9.75 14.00 = pH + pOH 14.00 = pH + 4.25 [H+1] = 1.78 x 10-10 M [OH-1] = 5.62 x 10-5 M pH = 9.75 pH = -log[H+1] pOH= -log[OH-1] 10-9.75 = 1.78 x 10-10 M H+1 10-4.25 = 5.62 x 10-5 M OH-1 Another way to check on some of the answers is to take the calculated [H+1] x [OH-1] and make sure that you get something close to 1.00 x 10-14!! For example, 2a has an [OH-1] = 2.82 x 10-2 M and an [H+1] = 3.55 x 10-13 2.82 x 10-2 * 3.55 x 10-13 = 1.00 x 10-14 ! 3.) A 0.0500 M acetic acid solution is tested with a pH probe and found to have a pH of 3.03 at 25oC. Use this information to calculate Ka for acetic acid if acetic acid dissociates into H3O+1 and the acetate ion in water. HAc 0.0500 -x 0.0500 - x + H2 O X X X ↔ pH = -log(H+1) 3.03 = -log(H+1) 10-3.03 = [H+1] = 9.33 x 10-4 M [H3O+1] = 9.33 x 10-4 M = x [H3O+1]eq = 9.33 x 10-4 M [Ac-1]eq = 9.33 x 10-4 M [HAc] = 0.0500 – 0.000933 = 0.0491 M [H 3 O 1 ][Ac 1 ] [9.33 x 10 -4 ][9.33 x 10 -4 ] Ka = = = [HAc] [0.0491] 1.78 x 10-5 Ac-1 0 +x x + H3O+1 0 +x x 4.) Ascorbic acid (formula does not matter at this time!), better known as vitamin C, is found in citrus fruits. The acid has a Ka value of 1.0 x 10-5. Calculate the concentration of ascorbic acid, the ascorbate ion, and the H+1 (H3O+1) at equilibrium if you have a solution of 0.050 M ascorbic acid. Vit C 0.050 -x 0.050 - x Ka = + H2 O X X X ↔ [H 3 O 1 ][C 1 ] [x][x] = = 1.0 x 10-5 [0.050 - x] [VitC] Assume x is small!! Such that 0.050 –x ≈ 0.050 1.0 x 10-5 = [x][x] [0.050] 5.0 x 10-7 = x2 x = 7.07 x 10-4 M assumption check!! 7.07 x 10 4 x 100 = 1.41 % which is less than 5% 0.050 Vitamin C = 0.050 – 0.00071 ≈ 0.050 M C-1 = 7.1 x 10-4 M H3O+1 = 7.1 x 10-4 M ***Extra*** pH = -log(H+1) = -log(7.1 x 10-4) = 3.1 C-1 0 +x x + H3O+1 0 +x x 5.) Calculate the concentrations of H+1, HC2O4-1, and C2O4-2 in a 0.10 M H2C2O4 solution. Ka1 = 5.90 x 10-2 and Ka2 = 6.40 x 10-5. (HINT: you will need two separate ICE tables and TWO separate calculations. The first will use Ka1 and the second will use Ka2) H2C2O4 0.10 -x 0.10 - x + ↔ H2 O X X X H1C2O4-1 0 +x x -1 Ka1 = [H 3 O 1 ][HC2 O 4 ] [x][x] = = 5.90 x 10-2 [H 2 C 2 O 4 ] [0.10 - x] Assume x is small!! Such that 0.10 –x ≈ 0.10 [x][x] = 5.90 x 10-2 [0.10] x2 = 5.9 x 10-3 x = 0.077 M assumption check!! 0.077 x 100 = 76.8 % which NOT less than 5% 0.10 Must use quadratic!! [x][x] = 5.90 x 10-2 [0.10 - x] 5.9 x 10-3 – 5.9 x 10-2x = x2 0 = x2 + 5.9 x 10-2 x – 5.9 x 10-3 x= x= x= 5.9x102 (5.9x102 ) 2 (4(1)(-5.9x10-3 )) b b 2 4ac = 2a 2(1) 5.9x102 (0.00348) (0.0236) 2 = 5.9x102 (0.0271) 2 5.9x102 0.1646 5.9x102 0.1646 or x = 2 2 x =0.053 or x = - 0.11 Your concentration (which is x!) cannot be negative! + H3O+1 0 +x x So x = 0.053 M [H2C2O4] = 0.10 – 0.053 = 0.05 M [HC2O4-1] = 0.053 M [H3O+1] = 0.053 M Because we have starting concentrations of HC2O4-1 AND of H3O+1 already present from the parent acid dissociation, we MUST put those starting amounts in our ICE table HC2O4-1 0.053 -x 0.053 - x + H2 O X X X -2 Ka2 = [H 3 O 1 ][C 2 O 4 ] -1 [H 1 C 2 O 4 ] = ↔ C2O4-2 0 +x x + H3O+1 0.053 +x 0.053 + x [x 0.053][x] = 6.40 x 10-5 [0.053 - x] Let’s assume that x is small – such that 0.053 –x ≈ 0.053 AND 0.053 + x ≈ 0.053 [ 0.053][x] = 6.40 x 10-5 [0.053] Look at how that math really simplifies things!! x = 6.40 x 10-5 M Check that assumption!! [ 6.40 x10-5 ] x100 = 0.12% which is less than 5% so assumption!! [0.053] [C2O4-2] = 6.40 x 10-5 M [H3O+1] = 0.053 + 6.40 x 10-5 = 0.053 M [HC2O4-1] 0.053 – 6.40 x 10-5 = 0.053 M And from our initial calculation H2C2O4 = 0.05 M 6.) List phosphoric acid and all of its conjugate base ions in order of increasing acidity and increasing basicity (HINT: think about what it means for a species to be an acid!! Think about what it means for a species to be a base!) H3PO4 H2PO4-1 + H+1 H2PO4-1 HPO4-2 + H+1 HPO4-2 PO4-3 + H+1 H3PO4 > H2PO4-1 > HPO4-2 for acid strength (PO4-3 is NOT an acid as it doesn’t have any H+1 to lose!) PO4-3 > HPO4-2 > H2PO4-1 > H3PO4 for base strength (H3PO4 could be a base and accept another H+1)