Intro to Acids and Bases
advertisement

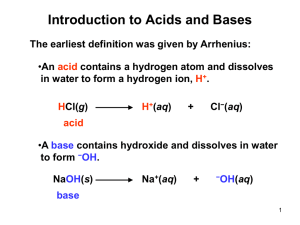
Acid-Base Chemistry Today’s Class •Definitions •Arrhenius Theory •Bronsted-Lowry Theory •Amphoteric/Amphiprotic Substances •Polyprotic Acids •Worksheet for practice 1661 - Robert Boyle Characterized acids as: corrosive, sour tasting, changes litmus from blue to red, and becomes less acidic when mixed with base. Characterized bases as: slippery feeling, turns litmus from red to blue, and becomes less basic when mixed with acid. Antoine Lavoisier Believed that all acids contained oxygen after studying several acids. Examples: Sulfuric acid (H2SO4) and Nitric acid (HNO3) 1811 - Humphry Davy Challenged Lavoisier’s belief that all acids contained oxygen by noting that Hydrochloric acid (HCl) did not contain oxygen but still was an acid. Other non-oxygen acids were also found HBr, HF, HI. •Arrhenius proposed that water could dissolve many substances into their individual ions. •He suggested that acids are compounds that contain hydrogen and can dissolve into water to release hydrogen ions into solution. •He defined bases as substances that dissolve in water to release hydroxide ions into solutions. •The Arrhenius definition also explains Boyle’s observation that acids and bases counteract each other. This idea that a base can make an acid weaker, and vice versa was later found to be called neutralization. Arrhenius Definition Acid = proton donor HA = H+ + A- Base = hydroxide donor BOH = B+ + OH- Dilemma: NH3 Dilemma • Though Arrhenius helped explain the fundamentals of acid/base chemistry, unfortunately his theories have limits. For example, the Arrhenius definition does not explain why some substances, such as common baking soda (NaHCO3) or ammonia(NH3), can act like a base even though they do not contain hydroxide ions. •Brønsted and Lowry separately proposed a new set of definitions for acids and bases which are known as either Brønsted acids and bases or Brønsted-Lowry acids and bases. •For Acids: Any substance that can donate a proton, H+ ion to a base. (Proton donor, or hydrogen-ion donor) •For Bases: Any substance that can accept a proton, H+ ion from an acid. (Proton acceptor or hydrogen-ion acceptor) The Brønsted-Lowry model of acid and bases helps develop the concept of Conjugate acid-base pairs. The part of the acid remaining when an acid donates a proton is called the conjugate base. The Acid formed when a base accepts the proton is called the conjugate acid. Strong acids have weak conjugate bases. Strong bases have weak conjugate acids. Bronsted-Lowry Definition Acid = proton donor Base = proton acceptor NH3 + H+ = NH4+ Amphoteric Substances • • An Amphoteric substance can act as either an acid or a base depending on their surroundings Examples include: Water, Al(OH)3 and Be(OH)2 Amphiprotic Substances •An amphiprotic substance can accept and donate protons •Examples include: Water, hydrogen carbonate(HCO3-) and hydrogen sulfate(HSO4-) Polyprotic Acids Are acids that are able to donate more than one proton per acid molecule • Example H2SO4- Sulfuric acid H2SO4 + H2O HSO4- + H3O+ Ka1= very large HSO4- + H2O SO42- + H3O+ Ka2=1.3X10-2 • Ka1 is greater than Ka2 • Polyprotic acids can be diprotic(able to donate 2 protons), triprotic(able to donate 3 protons) or can be more. • Conjugate acid-base example Consider the reaction of acid hydrogen fluoride with water. HF+H2O HF is donating its proton to the water molecule. In the reaction a proton is transferred from HF to H2O : HF+H2O H3O+ + FH2O is acting as a base in the reaction because it is accepting a proton. After it accepts the proton the water becomes a hydronium ion. It now has a proton, which it can donate, therefore it is an acid. Example continued • • • • When a base accepts a proton, it becomes an acid because it now has a proton that it can donate. When an acid donates a proton it becomes a base, because it now has room to accept a proton. These are what we call conjugate pairs of acids and bases So in this example the HF and F- are a conjugate acid-base pair and H2O and H3O+ are a conjugate acid-base pair. Another Example • H2SO4 + H2O • So in this example H2SO4 is the acid so it will get rid of a proton and form a conjugate base which is willing to accept a proton: HSO4H2O is acting as the base so when it accepts a proton it becomes a conjugate acid: H3O+ So the full reaction can be represented by: H2SO4 + H2O HSO4- + H3O+ • •