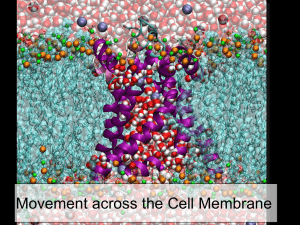
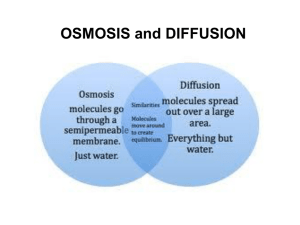
Guess What`s Behind the Box
advertisement

Guess What’s Behind the Box Next Slide Next Slide Remove Box Next Slide Remove Box Next Slide Remove Box Next Slide Remove Box Diffusion and Osmosis - A “Moving” Topic - Another Potato Demo... 1) Send two group members to obtain: - Two Beakers - Dry-Erase Marker - Triple Beam Balance Another Potato Demo... 1) Send two group members to obtain: - Two Beakers - Dry-Erase Marker - Triple Beam Balance 2) Get two slices of potato that are approximately the same size. Another Potato Demo... 3) Using the balance, determine the mass of each potato slice. Record in your table. 4) Using the dry-erase marker, label one beaker “water” and the other one “sugar solution.” 5) Using the beaker, measure 100mL of water for each beaker. Another Potato Demo... 6) Put a piece of paper on the balance. Measure out 30 grams of sugar and then add it to the “sugar solution” beaker. Mix. 7) Place one potato slice in each beaker. 8) Wait. Passive Transport • Cells maintain stable internal conditions by controlling what enters and leaves the cell. Passive Transport • Cells maintain stable internal conditions by controlling what enters and leaves the cell. What word do we use to describe “maintaining stable internal conditions?” (think 7 characteristics of life) Passive Transport • Cells maintain stable internal conditions by controlling what enters and leaves the cell. Homeostasis! Passive Transport • Cells maintain stable internal conditions by controlling what enters and leaves the cell. Homeostasis! • Some substances can cross the cell membrane without the cell having to use any energy. • This is known as passive transport. Passive Transport • There are three types of passive transport: 1) Diffusion 2) Osmosis 3) Facilitated Diffusion Diffusion • Diffusion is the simplest type of passive transport. Diffusion • Diffusion is the simplest type of passive transport. • Diffusion – the movement of molecules from an area of high concentration to an area of low concentration. Diffusion • Diffusion is the simplest type of passive transport. • Diffusion – the movement of molecules from an area of high concentration to an area of low concentration. • Concentration – Diffusion • Diffusion is the simplest type of passive transport. • Diffusion – the movement of molecules from an area of high concentration to an area of low concentration. • Concentration – the amount of a substance present. (Smelly) Example of Diffusion • Mrs. Shepherd sprays some cologne in the room. • Eventually, everyone in the room will be able to smell it. (Smelly) Example of Diffusion • Mr. Mathis sprays some cologne in the room. • Eventually, everyone in the room will be able to smell it. Why? (Smelly) Example of Diffusion • After the initial spray, there is a high concentration of the cologne in one spot. • The smell diffuses across the room, moving to areas where the concentration is low. Another Example of Diffusion • If you place a cube of sugar into a beaker of water, the concentration of sugar is highest at the bottom of the beaker. Another Example of Diffusion • If you place a cube of sugar into a beaker of water, the concentration of sugar is highest at the bottom of the beaker. • As the cube dissolves, the sugar molecules diffuse through the solution, moving toward the top of the beaker, where the concentration is lowest. Diffusion • Diffusion occurs because molecules are in constant motion. • Molecules tend to move from areas of high concentration to areas of low concentration. Diffusion • In the absence of other influences, diffusion will eventually lead to equilibrium. • At equilibrium, the concentration of molecules is the same throughout the space that the molecules occupy. Diffusion and the Cell Membrane • Cell membranes allow some molecules (but not others) to pass through. Diffusion and the Cell Membrane • If a molecule can diffuse through the cell membrane, it will diffuse until it reaches equilibrium. • Diffusion across the cell membrane is called simple diffusion. Diffusion and the Cell Membrane • Simple diffusion of molecules across the cell membrane depends on the size, charge and polarity of the molecule. Diffusion and the Cell Membrane • For example: – The cell membrane is made of phospholipids. – Oxygen and carbon dioxide are capable of dissolving in lipids, so can easily diffuse through the membrane. Diffusion and the Cell Membrane • For example: – Small molecules that can not dissolve in lipids may pass into the cell via pores in the membrane. Back to the Potato Lab 1) Remove the potato slices from the water solutions. 2) Pat them dry with paper towels and then measure the mass of each one on the triple beam balance. 3) Using the formula below, determine the percent change in mass and record in your table. Percent Change in Mass: ____(final mass – initial mass)___ initial mass Exit-Slip 1) Diffusion is the movement of molecules from an area of ____ concentration to ____ concentration. a) High ; Low b) Medium ; High c) Low ; High d) High ; Medium 2) Diffusion is a type of _____ ______, because it requires no energy input from the cell. a) active transport b) molecular transport c) effective transport d) passive transport 3) Assuming the pink dots can pass through the cell membrane, in what direction will they move? a) Out of the Cell b) Remain Constant c) Into the Cell 4) The dots will move until they reach a state of ... a) Equality b) Equilibrium c) Homeostasis d) Concentration Bell-Ringer True or False 1) Diffusion is the movement of molecules from an area of low concentration to an area of high concentration. 2) Cells use diffusion in order to move important molecules, such as oxygen, in and out of themselves. 3) Cells must use ATP in order to power diffusion. Bell-Ringer True or False 4) In the picture to the right, the dots will diffuse into the cell. 5) The cell pictured below is at equilibrium. Osmosis • Osmosis is the movement of water from an area of high concentration to an area of low concentration. • In other words, osmosis is the diffusion of water. The Direction of Movement • The direction of osmosis depends on the concentration of molecules dissolved in water. • We use three important terms to describe solutions: – Hypotonic – Hypertonic – Isotonic Hypotonic • A solution is hypotonic when the concentration of molecules outside the cell is lower than the concentration of molecules inside the cell. Hypotonic • To determine how water will move, we must first determine the concentration of the water. • Assume that each solution is made up only of water and the molecule dissolved in it. Hypotonic • To determine the concentration of water, subtract the concentration of the molecules dissolved in it from 100% Hypotonic • To determine the concentration of water, subtract the concentration of the molecules dissolved in it from 100% What is the concentration of water inside the cell? Hypotonic • To determine the concentration of water, subtract the concentration of the molecules dissolved in it from 100% 100% - 5% =95% Water Hypotonic • To determine the concentration of water, subtract the concentration of the molecules dissolved in it from 100% What is the concentration of water outside the cell? Hypotonic • To determine the concentration of water, subtract the concentration of the molecules dissolved in it from 100% 100% - 1% =99% Water Hypotonic • To determine the concentration of water, subtract the concentration of the molecules dissolved in it from 100% How will the water move? Hypotonic • To determine the concentration of water, subtract the concentration of the molecules dissolved in it from 100% Into the Cell! Hypotonic • When a solution is hypotonic to the cell, water will diffuse from outside the cell into the cell. • This will cause the cell to swell. Hypertonic • A solution is hypertonic when the concentration of molecules outside the cell is higher than the concentration of molecules inside the cell. Hypertonic What is the concentration of water inside the cell? Hypertonic 100% - 7% = 93% Water Hypertonic What is the concentration of water outside the cell? Hypertonic 100% - 10% = 90% Water Hypertonic How will the water move? Hypertonic Out of the cell! Hypertonic • When a solution is hypertonic to the cell, water will diffuse from inside the cell out into the solution. • This will cause the cell to shrivel. Isotonic • A solution is isotonic when the concentration of molecules outside the cell is the same as the concentration of molecules inside the cell. Isotonic What is the concentration of water inside the cell? Isotonic 100% - 10% = 90% Water Isotonic What is the concentration of water outside the cell? Isotonic 100% - 10% = 90% Water Isotonic How will the water move? Isotonic In and out of the cell at equal rates Isotonic • When a solution is isotonic to the cell, water will move both in and out of the cell at the same rate. • The cell will show no change. -Tonic Pictures • Pick an object and sketch it in three different environments: Isotonic, Hypotonic and Hypertonic • Be sure to show how would these three environments change this object. Exit Slip Match the words on the left to the corresponding picture on the right. 1) Hypotonic 2) Hypertonic 3) Isotonic A B C Bell-Ringer 1) What is the concentration of water outside the cell? 2) What is the concentration of water inside the cell? 3) How will the water move? 4) The solution is ______tonic Observing Osmosis Observing Osmosis... 1) Send one group member to obtain: - A Glass Slide and Cover - Small piece of Red Onion 2) Tear off a thin slice of the red onion skin. Place the skin and a drop of water on the slide. Cover with the cover slip. Observing Osmosis... 3) Observe the onion cells at 4x, 10x and 40x magnification. Sketch the cells. 4) After everyone in the group has sketched the cells, remove the cover slip and use a paper towel to dry off the onion and slide. 5) Place two drops of salt water (front desk) on the onion skin and re-cover. 6) Repeat step #3. 7) Answer questions. Exit-Slip 1) Osmosis is, specifically, the diffusion of... a) Ions b) Lipids c) Molecules d) Water 2) The cells to the right are mostly likely found in a ______ solution. a) Hypertonic b) Hypotonic c) Isotonic 3) In the example to the right, water will move... a) Out of the cell b) Into the cell c) In and Out of the cell at the same rate 4) After the water moves, the cell will look like which picture below?