13.& 13.2 - FIRST ROW d
advertisement

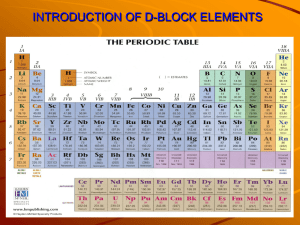
13.1 Periodicity First-Row d-block Elements(HL) *d-block elements = ____________ elements = Groups ____-____ *transition elements defined elements that contain an _______________ d- level of electrons in one or more of their _____________ states (e.g. For the first row of transition elements it is the _____ sub-level that is incomplete.) REVIEW: Complete the following table. ELEMENT / ION FULL e- configuration CONDENSED econfiguration ORBITAL DIAGRAM VANADIUM NICKEL NICKEL(II) CHROMIUM CHROMIUM(III) COPPER COPPER(II) *ZINC *ZINC(II) ION *Why is Zinc not classified as a transition metal? Zinc has completely ___________ d-orbitals in both its neutral and + 2 oxidation state. Sc Ti V Cr Mn Fe d-block elements transition elements Ni Co Cu Zn X CHARACTERISTIC PROPERTIES OF TRANSITION ELEMENTS: PROPERTY #1 Variable ______________________ States This simply means that most transition metals can form multiple ____________; i.e. have more than one valence. *ALL transition elements can show an oxidation number of _____. Examples: (In data booklet, p.14) Element Oxidation States Chromium, Cr +2, +3, +6 Manganese, Mn +2, +4, +7 Iron, Fe +2, +3 Copper, Cu +2, +1 Mercury, Hg +2, +1 Tin, Sn +2, +4 Explanation Compare the successive IE patterns of the d-block metals to the s-block metals. (See graph.) +2 all lose ___ electrons from the ___ orbital (except Cu/Cr – one lost from ____ ; another from ___) +3, +4(rare) ionization energies allows for the removal of up to two more electrons from _____ > +4 usually not found as the free metal ion / usually covalently bonded or as the oxyanion Examples: CrCl3, Cr2O72-, MnO2, MnO4-, Fe2O3, Cu2O 1 PROPERTY #2 Existence of _______________________Compounds Transition metal compounds and ions are often coloured. The explanation for this property will be considered in the next section (section 13.2 notes). Examples: KMnO4 purple [Mn(H2O)6]2+ Faint pink K2Cr2O7 Orange [Cr(H2O)6]3+ Green CuSO4•5H2O Blue [NH4]2[Fe(H2O)6][SO4]2 Pale green *N.B: Compounds of zinc(II) are usually _________________, unless the ligands in the complex have a chromophore; that is, a group of atoms that can absorb frequencies in the visible part of the EMR spectrum. PROPERTY #3 Complex ________ Formation IMPORTANT DEFINITIONS: (i) complex ion A complex ion consists of a ___________________ metal ______ surrounded by ______________. (ii) ligand an _______, _______________or______ containing a __________ pair of ________________ that bonds to the transition metal ion via ___________________ bonding. *Common EXAMPLES of ligands: (iii) coordinate covalent bond (a.k.a. dative covalent bond) both of the electrons in the covalent bond come from the __________ atom (unlike typical covalent bonding where the shared pair of electrons originate from ________ atoms.) *EXAMPLES OF SPECIES WITH COORDINATE BONDING: Hydronium, H3O+ Ammonium, NH4+ SPECIES LEWIS STRUCTURE Carbon monoxide, CO (iv) coordination # the number of _______pairs (from the __________) bonded to the _______________ ion (i.e. the total number of coordinate bonds present in the complex.) COMPLEX ION FORMATION (EXAMPLES) *Positive metal ions __________ ligands well, because of their _______ size and _____ charge densities. 1) Fe3+ + 6H2O ==== 2) Fe3+ + 6CN- ==== 3) Cu2+ + 4Cl- ==== 4) Ag+ + 2NH3 ==== 2 Complex 1) 2) 3) 4) Coordination # Shape Drawing Shape Name CLASSIFICATION OF LIGANDS (DENTICITY): A. MONODENTATE Ligands able to form only ____ coordinate bond with a ___________ ion contain single donor atom (donor of an electron pair) have one lone pair contributing to the coordinate bond in a complex examples: _____, _______, halides such as _______ B. POLYDENTATE Ligands (a.k.a CHELATE ligands) can form two or more coordinate bonds with a _____________ ion have two or more donor atoms that form __________________ bonds with a _________________ metal centre Examples: - 1,2-ethanediamine (en) (H2NCH2CH2NH2) - denticity = __________________ - a.k.a. ethylenediamine - ethanedioate (ox) (C2O42-) - denticity = ___________________ - a.k.a. oxalate - ethylenediaminetetraacetate, (EDTA)4- denticity = ___________(can form up to ___ coordinate bonds) EDTA Uses: -lead poisoning treatment: removes lead from blood -Chelation therapy: treatment for atherosclerosis (“hardening of the arteries) via decreasing calcium deposits in blood / atherosclerotic tissue -water softening: removes calcium and magnesium ions -food preservation: acts as “scavenger” for metal ions that catalyze food spoilage reactions -art restoration -cosmetic preservative 3 PROPERTY #4 __________________ Properties (The mechanisms of action will not be assessed.) A catalyst is a species that increases the rate of reaction by providing an alternate pathway with ____________activation energy; they are reaction _______________________ that are _____________________(chemically unchanged) at the _____of the reaction. Many transition metals are catalysts because of either their ability to form multiple _________________ states and/or the ability to provide __________________ filled d-orbitals to ___________________ bond to a reactant species. TWO TYPES OF CATALYSTS: 1) Heterogeneous – catalysts in a _____________ phase than the substances involved in the reaction How do they work? Heterogeneous catalysts ________________ reactant molecules onto the __________ of the metal. Transition metals are good at ___________ small molecules which makes them good __________________ catalysts. (*Definition of adsorb to ___________ on a _____________ in a condensed layer) 2) Homogeneous – catalysts in the _______________ phase as the substances involved in the reaction Examples: Reaction Catalyst Haber Process: Contact Process: Decomposition of hydrogen peroxide Hydrogenation of alkenes and oils BIOLOGICAL CATALYSTS (ENZYMES) Example: HEME - the _________ centre of hemoglobin (the protein that transfers oxygen in the blood) - vibrant red colour of blood stems from heme HEME OXYHEMOGLOBIN 4 PROPERTY #5 __________________ Properties *Paramagnetic materials contain __________________ electrons that behave as tiny magnets and are ______________ by an external magnetic field *Diamagnetic materials do not contain unpaired electrons and therefore are ______________ by external magnetic fields SUMMARY of TRANSITION METAL PROPERTIES: Property Reason 1) variable _______________ states (multiple loss of 4s2 electrons to give 2+ ions and loss of ions) other 3d electrons to give stable configurations 2) _______________ compounds partially filled d-orbitals 3) complex __________ formation partially filled split d-orbitals 4) _________________ properties partially filled d-orbitals 5) ________________ properties Many (e.g. oxidation state, coordination number, geometry of complex) 13.2 COLOURED COMPLEXES *Compounds of transition metals are usually _____________________. That means that there is an interaction with ________________. Some colours are ____________________, and some colours(the ______________________ ones) are not…they are transmitted. So why are some wavelengths (or frequencies, or energies, or colours) absorbed? In isolated atoms, d-orbitals have the __________ energy (they are degenerate) but in a complex ion, they split into two sublevels – two of the orbitals, dz2 and dX2-Y2, are raised to a higher potential energy (collectively referred to as eg), and the other three: dxy, dxz and dyz (collectively referred to as t2g) are lowered. Now remember that the transition metal behavior rests upon the presence of at least one __________________ filled d-orbital. This means there is the possibility for a lower level __________________to ______________some energy and be promoted to a __________________ d-orbital energy level. The energy difference between split d-orbitals is the same amount of energy as visible light. We observe the ________________________ colour to that absorbed (i.e. we observe the colour/frequencies that are not absorbed). *Examples: COMPLEX Frequencies Absorbed Frequencies Transmitted Colour of complex (Complementary to those absorbed) [Cu(H2O)6]2+ Orange [Ti(H2O)6]3+ Yellow-green *d-orbital “splitting”: *Why are compounds of zinc, scandium, copper(I) usually colourless? 5 *Note that the frequency of the light absorbed by the d-to-d electron transition is related to the ∆E in: E hf hc FACTORS THAT AFFECT THE SIZE OF ΔO, (The Crystal Field Splitting Energy…for Octahedral Complexes) FACTOR 1) identity of the metal ion 2) oxidation state of the metal ion 3) nature of the ligands Description In general, ΔO increases descending a group with a metal in the same oxidation state. For a given metal, ΔO increases as the oxidation state increases. Increase charge density = increase splitting *Spectrochemical series (in data booklet p. 14): I− < Br− < S2− < Cl− < F− < OH− < H2O < SCN− < NH3 < CN− ≈ CO 4) geometry of the complex ion An octahedral geometry will the splitting differently than a tetrahedral geometry, for example. *THE SPECTROCHEMICAL SERIES Ligands can be arranged into a spectrochemical series. The one provided is found in your data booklet on page 14. As we move from left to right along the series, the respective ligands increase the magnitude of ΔO (i.e., the amount of d-orbital splitting) more and more. The reason they split is because of the electrostatic interactions between the electrons of the ligand and the lobes of the d-orbital. I− < Br− < S2− < Cl− < F− < OH− < H2O < SCN− < NH3 < NO2- < CN− ≈ CO Ligands are classified as being either strong-field ligands or weak-field ligands: M2+ M3+ Strong-field Ligands to right of NO2 Ligands to right of H2O ligands Weak-field Ligands to left of NO2Ligands to left of H2O ligand Resulting electron configuration Spin-paired Spin-free Example illustrating the difference between the spin-paired and spin-free configurations: (i) [Fe(H2O)6]2+ Spin-free (ii) [Fe(CN)6]3- Spin-paired For more details regarding CFT go to: 1. http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Crystal_Field_Theory/Crystal_Field_Theory#Octahed ral_Complexes 2. https://www.youtube.com/watch?v=pZEjVRqe-N4&feature=youtu.be (MIT lecture) 6 PRACTICE PROBLEMS 1. For the complex K3[Fe(ONO)6], deduce: a) The oxidation state of the transition metal in the complex. b) The condensed electron configuration of the transition metal in this oxidation state. c) The coordination number of the metal in the complex. d) The stereochemistry (geometry / shape) of the complex. e) The charge on the complex. 2. Ni(ClO4)2 reacts with water to form the complex ion [Ni(H2O)6][ClO4]2. a) Explain this reaction in terms of electron pair transfers, and outline how the bond is formed between the ligand and the transition metal. b) What is the denticity of the ligand? c) What is the geometry of the cationic complex? d) What is the coordination number of the transition metal? 3. Consider the complex [Ni(NH3)6]Cl2. a) Deduce the condensed electron configuration of the transition metal in its associated oxidation state in the complex. b) State the geometry of the transition metal complex and draw a diagram of the complex. c) Identify the nature of the bonding between the ligand and the transition metal ion in the complex. d) State the denticity of the ammonia ligand. e) Draw a diagram showing the splitting of the d-sublevel. Label the orbitals involved and populate each of the orbitals with electrons. f) Explain whether the complex is paramagnetic or diamagnetic, 7