Intermolecular Forces
advertisement

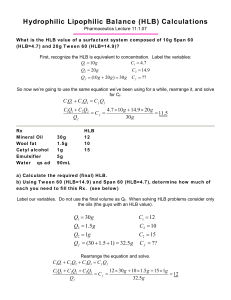
Instructor: Rong Cui, phd, 231 Pharmacy Bldg., 7-3255 Topics to be covered: Interfacial phenomena Surfactants Micelles Emulsions Introductions to polymers, rheology, and colloids Ointments Topical drug delivery Transdermal drug delivery. Books referred: Pharmaceutical Dosage forms and drug delivery systems, Ansel’s et al. Pharmaceutics: the science of dosage form design, ME Aulton Comprehensive pharmacy review, 5th ed., by Shargel et al. Physical pharmacy and pharmaceutical sciences, 5th ed. Sinko et al. The theory and practice of industrial pharmacy, 3rd ed., Lachman et al. Handbook of pharmaceutical excipients, 2nd ed., Wade and Weller Applied therapeutics, 8th ed., Koda-Kimble et al. Polymer process engineering, EA Gruke Remington: the science and practice of pharmacy, 21st ed. Goodman and Gilman’s Pharmacology, 9th ed. Journal of Pharmaceutical Sciences Transdermal drug delivery, 2nd ed., Guy and Hadgraft Interfacial phenomena and surfactants • • • • • • Molecule interaction Surface and interfacial tensions Surfactants Micelles HLB systems Other applications of surfactants Intermolecular Forces Ionic interactions Hydrogen bonds Dipole-dipole interactions London dispersion forces (van der Waals forces) 1,000 100 10 1 Hydrogen bond Hydrogen bonding occurs when a hydrogen atom is covalently bound to a small highly electronegative atom such as nitrogen, oxygen, or fluorine. The result is a dipolar molecule. The hydrogen atom has a partial positive charge δ+ and can interact with another highly electronegative atom in an adjacent molecule (again N, O, or F). This results in a stabilizing interaction that binds the two molecules together. An important example is water. Dipole-dipole interactions • Dipole-dipole interactions are the forces that occur between two molecules with permanent dipoles (spatially oriented δ+ within a molecule). These work in a similar manner to ionic interactions, but are weaker because only partial charges are involved. An example of this can be seen in hydrochloric acid: Van der Waals force • Also called London forces or instantaneous dipole effects (spatially variable δ+) these involve the attraction between temporarily induced dipoles in nonpolar molecules (often disappear within a second). Interfacial Phenomena Water strider, from Wikipedia Surface tension is an effect within the surface layer of a liquid that causes the layer to behave as an elastic sheet. Surface and interfacial tension • Surface tension = tendency of liquids to reduce their exposed surface to the smallest possible area. Work = g ΔA Work required to increase the surface area A of a liquid by 1 area unit. mN/m = dynes/cm Interfacial tension • At interfacial region/boundary/interface between two immiscible liquids or liquid-solid. Interfacial tension Surface active agents • Any substance that when dissolved in water or an aqueous solution reduces its surface tension or the interfacial tension between it and another liquid. Also called surfactant • Surfactants are usually organic compounds that are amphipathic, meaning they contain both hydrophobic groups (their "tails") and hydrophilic groups (their "heads"). Therefore, they are typically sparingly soluble in both organic solvents and water. Requirements for surfactants Classification of surfactants • Anionic • Cationic • Zwitterionic (amphoteric) • Non-ionic Anionic surfactants Laureth sulfate Soaps = salts of fatty acids • Soft soap: positive ion is univalent, such as Na+, K+, and NH4+. • Hard soap: positive ion is divalent, such as Ca++, Mg++ Cationic surfactants CTAB Non-ionic surfactants SPAN® SPAN Tween & poloxmers Structure of Tweens How do surfactants decrease surface tension? Surfactants decrease surface tension Effect of surfactants on the properties of a liquid • Osmotic pressure increases and reaches constant • Detergency ability increases sharply • Light scattering becomes significant • Surface tension decreases and reaches a constant (critical micelle concentration) Micelle A micelle is an aggregate of the surfactant molecules dispersed in a liquid colloid. Colloid = a kind of dispersed system with particle size ranging from 1 nm to 0.5 mm. Aggregation number Cholesterol-bearing pullulan selfaggregates (micelles) Micelle • Critical micelle concentration The concentration of surfactant at which micelles form. • Micelle aggregation number The number of surfactants that aggregate to form a micelle. • CMC of the mixture of two surfactants 1/CMC = f1/CMC1 + f1/CMC2 (f = mole fraction) Micelles are not a solid particles. The individual molecules in the micelles are in dynamic equilibrium with monomers in the bulk and at the interface. CMC and aggregation # of some surfactants Factors affecting CMC and micelle size • Structure of hydrophobic group • Nature of hydrophilic group • Nature of counter ions • Addition of electrolytes to ionic surfactants decreases CMC and increase size • Effect of temperature Solubilization • The ability of the micelles to increase the solubility of materials that are normally insoluble or only slight soluble in the dispersion medium. Systems of hydrophile-lipophile classification • Hydrophilic-lipophilic balance The higher the HLB, the more hydrophilic. • Required HLB (RHLB) values for emulsion. HLB system HLB of blending of surfactants • HLB values are additive If 20 mL of an HLB of 9.0 are required, what will be the ratio of two surfactants (with HLB values of 8 and 12) in the blend? HLB value of surfactant A = 8 HLB value of surfactant B = 12 Let the weight fraction of A = x; the weight fraction of B will be (1-X). 8X + 12 (1-X) = 9; 8X + 12 -12X = 9; -4X = -3 X = 3/4; 1-X = 1- ¾ = ¼ The ratio of A to B, A/B = 3:1 alligation B= 12 1 B= 15.6 9 A= 8 7.3 12 3 A = 4.7 4 3.6 10.9 B=¼ B = Tween 40 = 7.3/10.9 A = 3/4 A = Span 60 = 3.6/10.9 An example of HLB calculation • HLB of Tween 80 = 15 • HLB of Span 80 = 4.3 • We need 2 g of Tween 80 and Span 80 blend having a HLB value of 10.6. How much Tween 80 and Span 80 are needed? • Suppose we need X fraction of Tween 80; the fraction of Span 80 will be 1-X • 15X + 4.3 (1-X) = 10.6; X = (10.6-4.3)/(15-4.3) = 0.59 • Fraction of Span 80 = 1- 0.59 = 0.41 • Weight of Tween 80 = 2 * 0.59 = 1.18 g • Weight of Span 80 = 2 * 0.41 = 0.82 g HLB calculation B= 15 6.3 10.6 A = 4.3 4.4 10.7 B = Tween 80 = 6.3/10.7 A = Span 80 = 4.4/10.7 Applications of surfactants • • • • • • • • • • • • • • • Detergents Emulsifiers Paints Adhesives Inks Alveoli Wetting Ski Wax Snowboard Wax Foaming Defoaming Laxatives Agrochemical formulations Herbicides Insecticides Wetting agent • A wetting agent is a surfactant that, when dissolved in water, lowers the advancing contact angle, aids in displacing an air phase at the surface, and replacing it with a liquid phase. • Wetting agents can lower the contact angle. • Wetting agents should have an HLB value of 6-9. gc = 26-28 dynes/cm for real human skin Liquid Water Glycerin Diiodomethane Ethylene glycol Benzyl alcohol Mineral oil g (dynes/cm) 72.8 63.4 50.8 48.3 39.2 31.9 Cosθ 0.45 0.56 0.79 0.77 0.96 0.97 Detergent • Surfactants used for the removal of dirt. • Initial wetting of the dirt and of the surface to be cleaned; • Deflocculation and suspension; • Emulsification or solubilization of dirt; • Foaming of the agent for entrainment and washing away. Foaming and anti-foaming agents • Any solutions containing surfactants produce stable foams when mixed intimately with air. A foam is a relatively stable structure consisting of air pockets enclosed within thin films of liquid; the gas-in-liquid dispersion is being stabilized by a foaming agent. • Agents that can disrupt/break foams are anti-foaming agents.