Sem título de diapositivo
advertisement

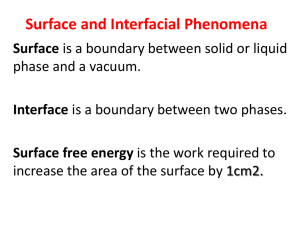
Surface and Interface Chemistry Thermodynamics of Surfaces (LG and LL Interfaces) Valentim M. B. Nunes Engineering Unit of IPT 2014 Adsorption in liquid surfaces Certain materials, such as fatty acids or alcohols, are soluble in water or in (e.g.) hydrocarbons. The nonpolar part is responsible for the solubility in "oil" and the polar part (-OH or -COOH) by solubility in water (Intermolecular forces). C2H5COOH C3H7COOH C4H9COOH Hydrophilic part Hydrophobic part Adsorption of molecules occurs (surfactants) in water-oil or water-air interfaces. ar The solutes that decrease the surface tension of a solvent are positively adsorbed in the interface, and the surface layers are enriched of solute. The solutes that increase surface tension tend to stay within the solution (e.g. Ionic salts) and are adsorbed negatively in the interface. The adsorption on solutions does not conduct in general to more than monolayers. The molecules that have a pronounced effect on surface tension are designated surfactants or surfactants. If the surface tension between two liquids is sufficiently reduced by the addition of surfactants it may form micro emulsions. GIBBS ADSORPTION ISOTHERM. The thermodynamic treatment of Gibbs allows the estimation of the adsorption on a liquid surface from the surface tension. Since the interface is a material system, with a given volume, they thermodynamic properties can be calculated. Let us consider a binary mixture containing ni moles of each component, and two homogeneous phases α and β, separated by an arbitrarily located interface. ni i A Surface excess concentration. From the laws of Thermodynamics: U TS pV A i ni i dU TdS S dT pdV V dp dA Ad i dni ni di i The combination of the 1st and 2nd laws gives: dU TdS pdV dA i dni i S dT V dp Ad ni di 0 i i At constant p and T, we have: ni d di i di A For a binary mixture (solvent + solute): d Ad A B dB Considering A = 0, d B dB Introducing the chemical potential, B RT ln aB d B RTd ln aB B We finally obtain: And for dilute solutions: 1 B RT ln aB cB B RT cB Gibbs Isotherm cB B RT cB 1 AB B,máx N A Monolayers Pockels and Rayleigh (1899): some poorly soluble substances spread on the surface to form films with thickness of a molecule monolayers This superficial film causes a lowering of the surface tension. It is determined by measuring the force, f, exerted in a calculated barrier to separate the region with film of the pure liquid. gas liquid + film liquid f 0 Considering now, kc 0 kc RT RT 0 Designating the difference of surface tensions 0 - by superficial pressure, , so that = 0 - , we obtain: ni A RT Rearranging: A ni RT Dividing both members of equation by the Avogadro constant: Am k BT The isothermal for the monolayer has the meaning of an equation of state (note the similarity to the equation of State of a perfect gas: pV = nRT) The type of isotherms (,Am) depends on the compounds structure. Materials with large surface activity tend to form micelles. The concentration from which the micelles are formed is called critical micelle concentration (c.m.c.) ~0 c c.m.c. concentration Surfactants forming micelles exhibit a solubility increase above a certain temperature – kraft point. This is due to the high solubility of the micelles. At temperatures below kraft point, the solubility of the surfactant is insufficient to form micelles. Kraft points for sodium alkyl sulfates Nº de átomos de carbono 10 12 14 16 18 Temperatura kraft / ºC 8 16 30 45 56 Spreading The work or energy of adhesion between two immiscible liquids is equal to the work necessary to separate a unit area of the liquid/liquid interface. By the Dupré equation: Wa For the cohesion work: Wc 2 Let us consider a drop of oil over water: AW OA oil air water OW We define the initial spreading coefficient in the following way: S WA OA OW The spreading occurs if S 0. Replacing in the Dupré equation we obtain: S WOW Wóleo