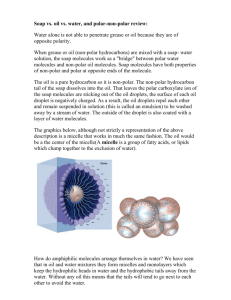
In Aqueous Environments
John Raia
Chem 4101
December 9th, 2011
The Problem
In the Deep Water Horizon catastrophe that occurred Spring
of 2010, over 1 million gallons of an undisclosed mixture of
sulfonated anionic surfactants (Corexit®) were sprayed across
the oil spill site to act as an oil dispersant agent.
Some specific anionic surfactants have known toxicities for
various marine species (especially green algae, sea turtles, and
dolphins). They are also known to have severe respiratory and
digestive issues in humans are suspected causers of human
endocrine disruption.
Over time the migration of the surfactants could effect
ecological systems many miles away from where they were
originally introduced. § § Kerr, R. A. A Lot of Oil on the Loose, Not So Much
to Be Found. Science 2010, 329, 734-735.
Hypothesis
It is crucial to be able to identify specific anionic surfactants
that are still present in aqueous environments.
Since migration of surfactants throughout aqueous sources is
an issue, quick and effective methods of sampling, detection
and characterizing surfactants is essential.
By monitoring the deaths of migrating aquatic species
(i.e. dolphins and sea turtles) found around coastal
shores, a direct correlation to the presence of anionic
surfactants in water in that area hopefully can be made,
and the contents can be analyzed both quantitatively
and qualitatively. º
ºNelson, Karen. One year later, Gulf oil disaster claims, questions unsettled
http://www.duluthnewstribune.com/event/article/id/196672/ (accessed
October 16th, 2011)
Classification of Anionic Surfactant Species
Classifications1
• Soaps : C H COO X
• Alkyl Sulfates (AS)
• Linear Alkylbenzene Sulfonates
(LAS) and Secondary Alkyl Sulfonates (SAS) : C H
• Alkylether Sulfates (AES): CnH2+ln
Dioctyl Sodium Sulfosuccinate
(DOSS)*
-
2n+1
n
n+1SO3
(OCH2CH2)n-OSO3X
•
•
•
•
444.216 Da
CAS #: 577-11-7
Molecular Formula: C20H37NaO7S
MP: 153–157 °C
• Fluorinated: C H
n
2n-1OPO(OH)O
-X
Approx. 1 million gallons of sulfonated
surfactant mixture dispersed in 6.43 E 17
gallons of marine water. (detection limit
from 1ppt to 3ppm) º
*Sigma-Aldrich.
<http://www.sigmaaldrich.com/catalog/ProductDetail.do?D7=0&N5=SEARCH
_CONCAT_PNO%7CBRAND_KEY&N4=D201170%7CALDRICH&N25=0&QS=
ON&F=SPEC >(accessed Oct 26, 2011).
Other standards for
comparison can be purchased
accordingly
-X
Sampling and Extraction
Sampling
Costal regions
Beached migrating aquatic species with known toxicities
Sampled radially, with constant distance
Positive sampling for anionic surfactants should be resampled at
varying depths
• Solid Phase Extraction (C18 cartridge)2
100 mL sample
of seawater
Condition w/
MeOH and H2O
Frozen with 4%
formaldehyde until
analysis
Possible Separation Methods
Method
Advantages
Disadvantages
Capillary electrophoresis2
•
Reduced demand for organic
solvents
Short time for analysis
Easy to carry out
•
Large sample volumes would
be hard to quantitatively
analyze LOD without
painstaking sample prep
Can be paired with tandem
MS
Lower concentrations of
single analyte can be
measured
Lower limit of detection
compared to HPLC
•
Limit capability for
compounds with low
volatility
Derivitization of analyte is
necessary
Apparatus is complicated and
can be more expensive than
HPLC
All groups of anionic
surfactants can be analyzed
Low volatile analyte can be
determined
Various methods of detection
are available for coupling
•
•
•
Gas Chromatography3
•
•
•
HPLC (Reverse Phase)1,5,6,7
•
•
•
•
•
•
•
Costly analysis (very pure
solvents)
Expensive equipment
Produces toxic wastes
Possible Detection Methods
Method
Advantages
Disadvantages
UV-Vis†
• Quick
• Inexpensive
• Limited usage with
specific conjugated
surfactants (e.g. LAS)
• AS and AES must be
coupled with
conjugating dyes to
emit detectable
wavelength
Mass Spec1
• provides specific
information about
MW and structure
• Specific tailoring for
analyte in question
• Many options (Ion
Trap, Triple Quad,
TOF… etc.)
• Expensive
• Many parameters for
consideration
• Destruction of sample
† Yuxiu
A. et al. Soft Matter. 2011, 7, 6873.
Method of Choice: HPLC-MS
Reversed Phase Column (C18)
Gradient elution for lowering time of detection
Ion Trap Mass Spec
Atmospheric Ionization Source with ESI
Full scan mode
Fragmentations from 75 to 800 m/z
LOD – 0.00021 ug/L (1ppt = 1 ug/L)1
http://www.forumsci.co.il/HPLC/system.gif
Equipment
Column: LiChristopher 100
RP-18 (Merck)(9)
250mm x 2mm and 3um
particle diameter
HPLC- Ion Trap MS (5)
Atmospheric pressure
Chemical Ionization
Negative Ion Quadrupole
Mode
LCQ Fleet Ion Trap Mass
Spectrometer from
ThermoScientific
Example Data
(4)
Conclusion
The methods that were proposed allow not only for the
detection and presence anionic surfactants at low
limits of detection that are needed for the study of
anionic surfactants in the gulf, but also allow for
differentiation of homologues as long as a standard
comparison is present.
References
(1) Olkowska, E.; Polkowska, Ż.; Namieśnik, J. Analytics of surfactants in the environment: problems and
challenges. Chem. Rev. 2011, 111, 5667-5700. (
(2) Riu, J.; Eichhorn, P.; Guerrero, J. A.; Knepper, T. P.; Barceló, D. Determination of linear
alkylbenzenesulfonates in wastewater treatment plants and coastal waters by automated solid-phase
extraction followed by capillary electrophoresis–UV detection and confirmation by capillary
electrophoresis–mass spectrometry. Journal of Chromatography A 2000, 889, 221-229.
(3) Alzaga, R.; Peña, A.; Ortiz, L.; Marı́a Bayona, J. Determination of linear alkylbenzensulfonates in
aqueous matrices by ion-pair solid-phase microextraction–in-port derivatization–gas chromatography–mass
spectrometry. Journal of Chromatography A 2003, 999, 51-60.
(4) Lara-Martín, P. A.; Gómez-Parra, A.; González-Mazo, E. Simultaneous extraction and determination of
anionic surfactants in waters and sediments. Journal of Chromatography A 2006, 1114, 205-210.
(5) Petrovic, M. Determination of anionic and nonionic surfactants, their degradation products, and
endocrine-disrupting compounds in sewage sludge by liquid chromatography/mass spectrometry. Anal.
Chem. 2000, 72, 4560.
(6) Poppe, A. Negative-ion mass spectrometry. X. A spurious [CH5]- ion: problems with negative chemicalionization quadrupole instrument. Org. Mass Spectrom. 1986, 21, 59.
(7) Boiani, J. Spectator ion indirect photometric detection of aliphatic anionic surfactants separated by
reverse-phase high-performance liquid chromatography. Anal. Chem. 1987, 59, 2583.
(8) An, Y.; An, Y. Disassembly-driven colorimetric and fluorescent sensor for anionic surfactants in water
based on a conjugated polyelectrolyte/dye complex. Soft matter 2011, 7, 6873.
(9) LiChrospher® 100 RP-18 and RP-18 Endcapped | Merck Chemicals International http://www.merckchemicals.com/lichrospher-100-rp-18-and-rp-18-endcapped/c_DMOb.s1LSAoAAAEWsOAfVhTl (accessed
11/11/2011, 2011).