WATER AND SOLUTIONS
advertisement

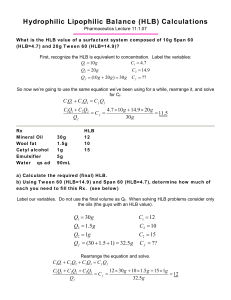
SURFACTANT S IN SOLUTION Classification of Surfactants • • • • Anionic Cationic Zwitterionic Nonionic O S - + O Na O Sodium dodecylsulfate (SDS) + N Br- Cetylpyridinium bromide O O O OCH2CH2N(CH3)3+ P OO O Dipalmitoylphosphatidylcholine (lecithin) O O O Polyoxyethylene(4) lauryl ether (Brij 30) O OH Surfactant Aggregates Unimers Normal micelles cylindrical spherical Inverted hexagonal phase Reverse micelles Bilayer lamella 4 nm Critical Micelle Concentration CMC 14 12 10 8 6 CMC 4 2 0 0 1 Surfactant concentration • Below CMC only unimers are present • Above CMC there are micelles in equilibrium with unimers Solution Properties 14 12 10 8 6 4 2 0 14 12 Concentration unimers 10 8 6 4 2 0 CMC micelles 0 Surfactant concentration1 14 Isc12 10 8 6 4 2 0 Osmotic pressure CMC 0 Surfactant concentration1 Light scattering CMC 0 Surfactant concentration1 Solubilization • Spontaneous transfer of a compound insoluble in the bulk solvent into solution due to incorporation into the surfactant micelles Normal micelles non-polar compound amphiphilic compound Reverse micelles polar compound Solubility Effects • Solubility of a poorly soluble compound increases as a result of solubilization in the micelles Solubility 14 12 10 8 6 CMC 4 2 0 0 1 Surfactant concentration An example of an HLB value calculation • BRIJ ® 98 INCI name : oleth-20 is a 20 mole ethoxylate of oleyl alcohol • calculate the molecular weight of the 20 moles of ethylene oxide ( one mole ETO =44 ); 20 x 44 = 880 • add this number to the molecular weight of the oleyl alcohol; 880+ 270 = 1150 ( the mol. wt of BRIJ 98 ) • What percentage of 1150 is 880 ? 880/1150 = 76.5% • 76.5% divided by 5 = 15.3 • 15.3 is the HLB value of BRIJ 98 Important to remember ! HLB and Use of Surfactants Amphiphilic surfactants are characterized by the hydrophilic-lipophilic balance (HLB): a relative ratio of polar and non-polar groups in the surfactant • • • • • • HLB ca. 1 to 3.5: Antifoams HLB ca. 3.5 to 8: Water-in-Oil Emulsifiers HLB ca. 7 to 9: Wetting and spreading agents HLB ca. 8 to 16: Oil-in-Water Emulsifiers HLB ca. 13 to 16: Detergents HLB ca. 15 to 40: Solubilizers Required HLB HLB needed for emulsification of the oil phase. If there are several oil ingredients the required HLB is calculated as a sum of their respective required HLB multiplied by the fraction of each. • Calculate the required HLB for the oil phase of the following o/w emulsion: cetyl alcohol 15 g., white wax 1g. Lanolin 2 g, emulsifier (q.s.), glycerin 5 g. water 100 g. Cetyl alcohol White wax Lanolin Total required HLB Required HLB (from reference) 15 x 12 x 10 x Fraction 15/18 1/18 2/18 12.5 0.7 1.1 14.3 HLB of Surfactant Blend Surfactant blends are commonly used to obtain desired emulsifying properties. • • • • Span 60 (HLB = 4.7) Tween 60 (HLB = 14.9) Span 80 (HLB = 4.3) Tween 80 (HLB = 15.0) What is the HLB of the mixture of 40 % Span 60 (HLB = 4.7) and 60 % Tween 60 (HLB = 14.9)? Sorbitan monostearate is an ester of sorbitol and stearic acid (synthetic wax) Polyoxyethylenesorbitan monooleate; Sorbitan monooleate ethoxylate HLB of mixture: 4.7 x 0.4 + 14.9 x 0.6 = 10.8 In what proportion should Span 80 (HLB = 4.3) and Tween 80 (HLB = 15.0) be mixed to obtain “required” HLB of 12.0? 4.3.(1-x) + 15.x = 12 x = 0.72 72 % Tween 80 and 28 % Span 80 Glycerol Monostearate HLB value of 3.6~4.2 dissolves in hot grease, paraffine, ethanol, chloroform, acetone and aether, the material is widely used when producing of chocolate, margarine, ice cream, skin care balsam, cold cream, hair oil and drug ointment, also lubricant for plastic processing Shampoo Ingredients • • • • • • • • • Isobutane Cyclomethicone Aluminum Chlorohydrate Propylene Glycol Dicaprylate/Dicaprate Lichen extract Fragrance Quaternium-18 Silica Dimethiconol cyclomethicone Propylene Glycol Dicaprylate/Dicaprate Facial Cleanser Aqua/Water Palmitic acid Myristic acid Lauric acid Stearic acid Potassium hydroxide PEG-7 Glyceryl cocoate PEG-150 Distearate Glycerin Sodium laureth sulfosuccinate Limonene Linalool Zinc PCA Propylparaben Propylene glycol Disodium EDTA NaCl Propylene glycol propyl ester of p-hydroxybenzoic acid = propylparaben zinc salt of 2-Pyrrolidone-5-Carboxylic Acid PEG-7 Glyceryl cocoate Polyoxyethylene glycol glyceryl cocoate (monococoate). PEG-150 Distearate Polyethylene glycol diester of stearic acid. Sodium laureth sulfosuccinate Cream