Water-Soluble, Monolayer-Protected Quantum Dots
Joseph A. Giesen, Elizabeth M. Henry, April D. Dale, Adrienne C. Borchardt, and Deon T. Miles Department of Chemistry
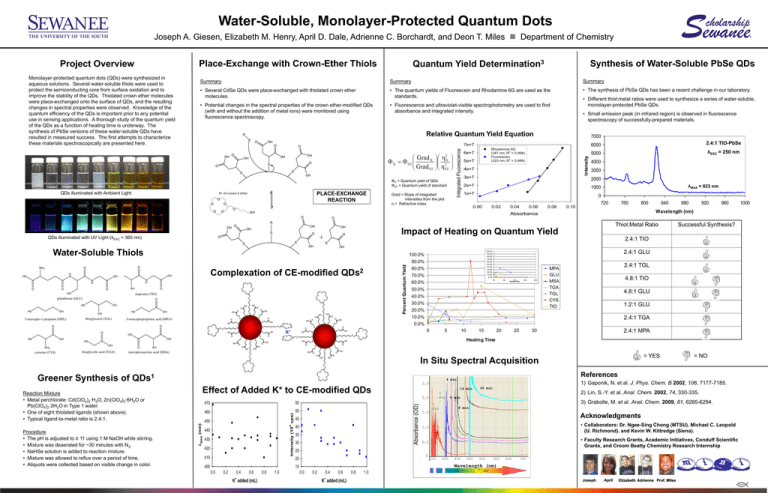
Synthesis of Water-Soluble PbSe QDs
Summary
Summary
Summary
• Several CdSe QDs were place-exchanged with thiolated crown ether
molecules.
• The quantum yields of Fluorescein and Rhodamine 6G are used as the
standards.
• The synthesis of PbSe QDs has been a recent challenge in our laboratory.
• Potential changes in the spectral properties of the crown ether-modified QDs
(with and without the addition of metal ions) were monitored using
fluorescence spectroscopy.
• Fluorescence and ultraviolet-visible spectrophotometry are used to find
absorbance and integrated intensity.
• Different thiol:metal ratios were used to synthesize a series of water-soluble,
monolayer-protected PbSe QDs.
• Small emission peak (in infrared region) is observed in fluorescence
spectroscopy of successfully-prepared materials.
Relative Quantum Yield Equation
7000
7e+7
ФX = Quantum yield of QDs
ФST = Quantum yield of standard
QDs illuminated with Ambient Light
PLACE-EXCHANGE
REACTION
R= 15-crown-5 ether
Grad = Slope of integrated
intensities from the plot
η = Refractive index
Rhodamine 6G
2
(347 nm; R = 0.998)
Fluorescein
2
(323 nm; R = 0.989)
6e+7
5e+7
Intensity
Monolayer-protected quantum dots (QDs) were synthesized in
aqueous solutions. Several water-soluble thiols were used to
protect the semiconducting core from surface oxidation and to
improve the stability of the QDs. Thiolated crown ether molecules
were place-exchanged onto the surface of QDs, and the resulting
changes in spectral properties were observed. Knowledge of the
quantum efficiency of the QDs is important prior to any potential
use in sensing applications. A thorough study of the quantum yield
of the QDs as a function of heating time is underway. The
synthesis of PbSe versions of these water-soluble QDs have
resulted in measured success. The first attempts to characterize
these materials spectroscopically are presented here.
Quantum Yield Determination3
Place-Exchange with Crown-Ether Thiols
Integrated Fluorescence
Project Overview
4e+7
3e+7
6000
2.4:1 TIO-PbSe
5000
λEXC = 250 nm
4000
3000
2000
2e+7
λMAX = 823 nm
1000
1e+7
0.00
0.02
0.04
0.06
0.08
0
720
0.10
760
800
840
880
920
960
Wavelength (nm)
Absorbance
Thiol:Metal Ratio
Successful Synthesis?
Impact of Heating on Quantum Yield
QDs illuminated with UV Light (λEXC = 365 nm)
2.4:1 TIO
Water-Soluble Thiols
2.4:1 GLU
100.0%
Complexation of CE-modified
Percent Quantum Yield
90.0%
2
QDs
80.0%
2.4:1 TGL
MPA
GLU
MSA
TGA
TGL
CYS
TIO
70.0%
60.0%
50.0%
40.0%
30.0%
20.0%
4.8:1 TIO
4.8:1 GLU
1.2:1 GLU
10.0%
2.4:1 TGA
0.0%
0
K+
5
10
15
20
25
2.4:1 MPA
30
Heating Time
= YES
In Situ Spectral Acquisition
Effect of Added
+
K
to CE-modified QDs
16 min
2.0
55
470
460
5
450
440
430
420
410
Absorbance (OD)
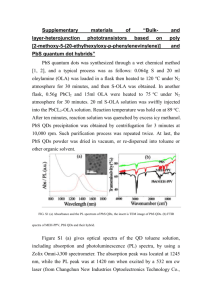
Procedure
• The pH is adjusted to ≥ 11 using 1 M NaOH while stirring.
• Mixture was deaerated for ~30 minutes with N2.
• NaHSe solution is added to reaction mixture.
• Mixture was allowed to reflux over a period of time.
• Aliquots were collected based on visible change in color.
1) Gaponik, N. et al. J. Phys. Chem. B 2002, 106, 7177-7185.
2.5
Intensity (10 cps)
Reaction Mixture
• Metal perchlorate: Cd(ClO4)2·H2O, Zn(ClO4)2·6H2O or
Pb(ClO4)2·2H2O in Type 1 water.
• One of eight thiolated ligands (shown above).
• Typical ligand-to-metal ratio is 2.4:1.
References
4 min
MAX (nm)
Greener Synthesis of
QDs1
50
45
40
35
30
0.2
0.4
+
0.6
K added (mL)
0.8
1.0
2) Lin, S.-Y. et al. Anal. Chem. 2002, 74, 330-335.
6 min
3) Grabolle, M. et al. Anal. Chem. 2009, 81, 6285-6294.
8 min
Acknowledgments
1.0
• Collaborators: Dr. Ngee-Sing Chong (MTSU). Michael C. Leopold
(U. Richmond). and Kevin W. Kittredge (Siena).
0.5
• Faculty Research Grants, Academic Initiatives, Conduff Scientific
Grants, and Croom Beatty Chemistry Research Internship
0
15
0.0
20 min
25
20
400
1.5
0
min
2
min
.
0.0
0.2
0.4
+
0.6
K added (mL)
0.8
1.0
= NO
350
400
450
500
550
600
650
700
Wavelength (nm)
Heating Time
Joseph
April
Elizabeth Adrienne Prof. Miles
1000