Extended abstract
advertisement

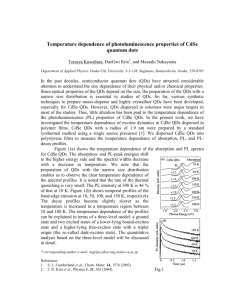
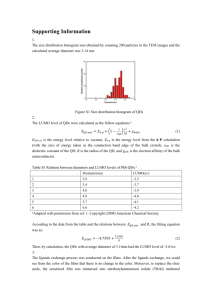
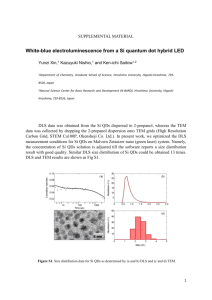
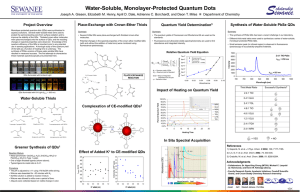
Synthesis of SiO2-coated CdSe/CdS/ZnS quantum dots nanohybrids Valentina V. Goftman, SSU - UGent Irina Yu. Goryacheva, SSU Quantum dots (QDs) are high-luminescence photostable nanoparticles with unique optical properties: opposite to known organic fluorescent dyes, QDs have a symmetric and narrow band which enables emission of pure color and broad absorption spectrum. These facts allow to excite different sized particle with a single wavelength. This makes QDs ideal labels for usage in biological and chemical detecting [1]. Weak points of QDs, which are restricted their application as bio- and analytical labels, are toxicity, water insolubility and absence of functional groups available for bioconjugation. To overcome these disadvantages multiple QDs were introduced into silica nanoshells. The versatility of silica shells in synthesis aspects as well as surface modifications offers a great advantage to the use of the obtained material in bioanalysis [2, 3]. The other reasons of using this method are anomaly high stability of covered particles, especially in aqueous media, easy regulation of the coating process, chemical inertness, controlled porosity, processability, optical transparency and, last but not least, low-cost reagents [4]. Amplification of analytical signal of the final hydrophilic hybrid QD@SiO2 can be achieved by encapsulation a multiple QD in one silica nanobead. The preparation of silica nanoparticles within microemulsions is a convenient route toward monodisperse particles of controllable size. This method allows to obtain both single QDs in silica spheres and multiple QDs in silica spheres by varying the water-to-surfactant molar ratio and time of reaction. Method of incorporation of multiple hydrophobic CdSe/CdS/ZnS QDs into SiO2 beads by a two-step microemulsion process was carried out. Hydrophobic CdSe/CdS/ZnS core–shell QDs with a peak wavelength of 615 nm and 625 nm were prepared via organic route by using oleic acid (OA) as a capping agent, shells of CdS and ZnS were grown by SILAR technology. 1-st step of synthesis: ligand exchange of oleic acid –capped CdSe/CdS/ZnS QDs, during which initial molecules of oleic acid removed from the QDs surface while molecules of 3mercaptopropyltrimethoxysilane (MPS) connect with it. 2-nd step of synthesis: transfer these silanized QDs into aqueous phase in the reverse micelle solution by adding of a small amount of water and stirring for a few hours. Then these silanized QDs assembled and subsequently encapsulated in a SiO2 shell by a reverse micelle synthesis During this procedure the photoluminescence of QDs was not significant changed. Size of nanohybrids was varied from 70 nm to 170 nm depending of size of initial QDs and synthesis condition (silanization reagent, time of reaction, water-to-surfactant molar ratio). 1. 2. 3. 4. Goryacheva I. Yu., Lenain P., DeSaeger S. Nanosized labels for rapid immunotests.// Trends in Analytical Chemistry. 2013. Vol. 46. P. 30 – 43. Tan W., Wang K., He X., Zhao X. J., Drake T., Wang L., Bagwe R. P. Bionanotechnology Based on Silica Nanoparticles.// Medicinal Research Reviews. 2004. Vol. 24. No. 5. P. 621 – 638. Knopp D., Tang D., Niessner R. Bioanalytical applications of biomolecule-functionalized nanometer-sized doped silica particles.// Analytica Chimica Acta. 2009. Vol. 647. P. 14-30. UV-VIS and Photoluminescence Spectroscopy for Nanomaterials Characterization. Editor Challa S.S.R. Kumar.// Springer-Verlag Berlin Heidelberg. 2013. P. 431 – 449.