Chapter 19 Carboxylic Acids
advertisement

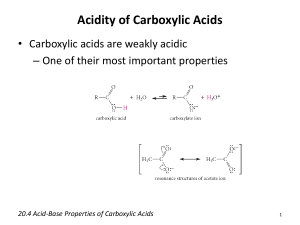
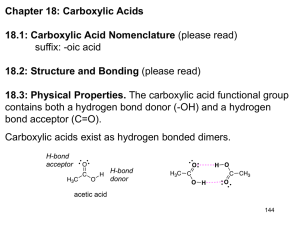
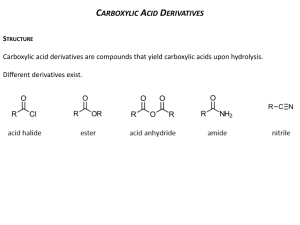
Carboxylic Acids Structure and Bonding Electron Delocalization R C •• • O• •• O •• R + C •• •– O• •• •• O •• H H Electron Delocalization R C •• • O• •• O •• R + C •• •– O• •• R •• O •• H + O •• H stabilizes C •• •– O• •• carbonyl group H Formic acid is planar O H C 120 pm H O 134 pm Carboxylic Acid Nomenclature NOMENCLATURE systematic IUPAC names replace "-e" ending of alkane with "oic acid" Systematic Name O HCOH methanoic acid O CH3COH O CH3(CH2)16COH ethanoic acid octadecanoic acid NOMENCLATURES common names are based on natural origin rather than structure Systematic Name Common Name O HCOH methanoic acid formic acid ethanoic acid acetic acid octadecanoic acid stearic acid O CH3COH O CH3(CH2)16COH NOMENCLATURE Systematic Name Common Name O CH3CHCOH OH 2-hydroxypropanoic acid O CH3(CH2)7 (CH2)7COH C H lactic acid C H (Z)-9-octadecenoic acid oleic acid Physical Properties Hydrogen-bonded Dimers O H O CCH3 H3CC O H O Acetic acid exists as a hydrogen-bonded dimer in the gas phase. The hydroxyl group of each molecule is hydrogen-bonded to the carbonyl oxygen of the other. Hydrogen-bonded Dimers Acetic acid exists as a hydrogen-bonded dimer in the gas phase. The hydroxyl group of each molecule is hydrogen-bonded to the carbonyl oxygen of the other. Solubility in Water carboxylic acids are similar to alcohols in respect to their solubility in water form hydrogen bonds to water H O H O H3CC H O H O H Greater acidity of carboxylic acids is attributed stabilization of carboxylate ion by inductive effect of carbonyl group O – RC O d+ resonance stabilization of carboxylate ion •• O •• RC •• – O •• •• •• – •O• • • RC O •• •• Dicarboxylic Acids Dicarboxylic Acids O HOC O COH 1.2 Malonic acid 2.8 Heptanedioic acid 4.3 O HOC(CH2)5COH Oxalic acid O HOCCH2COH O pKa O one carboxyl group acts as an electronwithdrawing group toward the other; effect decreases with increasing separation Reactions of Carboxylic Acids Acidity Reduction Esterification Reaction with Thionyl Chloride Reactions of Carboxylic Acids New reactions in this chapter a-Halogenation Decarboxylation But first we revisit acid-catalyzed esterification to examine its mechanism. Acidity of Carboxylic Acids Most carboxylic acids have a pKa close to 5. Carboxylic acids are weak acids but carboxylic acids are far more acidic than alcohols O CH3COH CH3CH2OH Ka = 1.8 x 10-5 pKa = 4.7 Ka = 10-16 pKa = 16 Substituent Effects on Acidity O X CH2COH X Ka pKa H 1.8 x 10-5 4.7 F 2.5 x 10-3 2.6 Cl 1.4 x 10-3 2.9 electronegative substituents increase acidity Salts of Carboxylic Acids Carboxylic acids are neutralized by strong bases O RCOH + stronger acid O HO– RCO– + H2O weaker acid equilibrium lies far to the right; K is ~ 1011 as long as the molecular weight of the acid is not too high, sodium and potassium carboxylate salts are soluble in water Micelles unbranched carboxylic acids with 12-18 carbons give carboxylate salts that form micelles in water O ONa sodium stearate (sodium octadecanoate) O – CH3(CH2)16CO Na+ Micelles O ONa nonpolar polar sodium stearate has a polar end (the carboxylate end) and a nonpolar "tail" the polar end is "water-loving" or hydrophilic the nonpolar tail is "water-hating" or hydrophobic in water, many stearate ions cluster together to form spherical aggregates; carboxylate ions on the outside and nonpolar tails on the inside A micelle a -Halogenation of Carboxylic Acids O R2CCOH + X2 H O R2CCOH + HX X analogous to a-halogenation of aldehydes and ketones key question: Is enol content of carboxylic acids high enough to permit reaction to occur at reasonable rate? (Answer is NO) Reactions O CH3CH2CH2COH O Br2 P CH3CH2CHCOH Br (77%) a-Halogen can be replaced by nucleophilic substitution Decarboxylation of Carboxylic Acids Simple carboxylic acids do not decarboxylate readily. O RH + CO2 RCOH But malonic acid does. O O HOCCH2COH 150°C O CH3COH + CO2 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Acyl Halides O RC X name the acyl group and add the word chloride, fluoride, bromide, or iodide as appropriate acyl chlorides are, by far, the most frequently encountered of the acyl halides Acyl Halides O acetyl chloride CH3CCl O H2C CHCH2CCl 3-butenoyl chloride O F CBr p-fluorobenzoyl bromide Acid Anhydrides O O RCOCR' when both acyl groups are the same, name the acid and add the word anhydride when the groups are different, list the names of the corresponding acids in alphabetical order and add the word anhydride Acid Anhydrides O O CH3COCCH3 acetic anhydride O O C6H5COCC6H5 benzoic anhydride O O C6H5COC(CH2)5CH3 benzoic heptanoic anhydride Some anhydrides are industrial chemicals O O O O CH3COCCH3 O Acetic anhydride Phthalic anhydride O O O Maleic anhydride Esters O RCOR' name as alkyl alkanoates cite the alkyl group attached to oxygen first (R') name the acyl group second; substitute the suffix -ate for the -ic ending of the corresponding acid Esters O CH3COCH2CH3 ethyl acetate O CH3CH2COCH3 methyl propanoate O COCH2CH2Cl 2-chloroethyl benzoate Esters are very common natural products O CH3COCH2CH2CH(CH3)2 3-methylbutyl acetate also called "isopentyl acetate" and "isoamyl acetate" contributes to characteristic odor of bananas Lactones Lactones Formed are cyclic esters by intramolecular esterification in a compound that contains a hydroxyl group and a carboxylic acid function Examples O HOCH2CH2CH2COH 4-hydroxybutanoic acid O + O H2O 4-butanolide IUPAC nomenclature: replace the -oic acid ending of the carboxylic acid by -olide identify the oxygenated carbon by number Common names b a O b g O g-butyrolactone a g O d O d-valerolactone Ring size is designated by Greek letter corresponding to oxygenated carbon A g lactone has a five-membered ring A d lactone has a six-membered ring Amides having an NH2 group O RCNH2 identify the corresponding carboxylic acid replace the -ic acid or -oic acid ending by amide. Amides having an NH2 group O CH3CNH2 acetamide O (CH3)2CHCH2CNH2 3-methylbutanamide O CNH2 benzamide Amides having substituents on N O RCNHR' O and RCNR'2 name the amide as before precede the name of the amide with the name of the appropriate group or groups precede the names of the groups by the letter N(standing for nitrogen and used as a locant) Amides having substituents on N O N-methylacetamide CH3CNHCH3 O CN(CH2CH3)2 N,N-diethylbenzamide O CH3CH2CH2CNCH(CH3)2 CH3 N-isopropyl-N-methylbutanamide Nitriles RC N add the suffix -nitrile to the name of the parent hydrocarbon chain (including the triply bonded carbon of CN) or: replace the -ic acid or -oic acid name of the corresponding carboxylic acid by -onitrile or: name as an alkyl cyanide (functional class name) Nitriles CH3C C6H5C N N CH3CHCH3 C N ethanenitrile or: acetonitrile or: methyl cyanide benzonitrile 2-methylpropanenitrile or: isopropyl cyanide