Structures and Types of Solids
advertisement

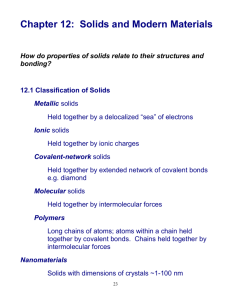
Structures and Types of Solids Two types of solids 1. Crystalline solids highly regular arrangement of their particles crystals- at microscopic level How are they crystals? Lattice - 3-D system of points indicating position of ions, atoms or molecules that make up the substance Unit cell Smallest repeating pattern of the latticeextend in all directions for the structure 3 types of unit cells A. Simple cubic polonium metal B. Body centered cubic Uranium metal C. Face centered cubic Gold metal Two types of solids 2. Amorphous solidsconsiderable disorder in their structure EX- glass- liquid that is “frozen in place” Crystalline solids?? How do we determine the structure of the solid? X-ray diffraction!! Bragg equation 1915 Noble prize in physics n = 2dsin where n= integer = wavelength of x-rays d= distance b/w atoms = angle of incidence Types of crystalline solids Easy to classify based on what particle is at lattice point Types of crystalline solids Explains why solids have different properties melting point conductivity ductility Three types 1. Ionic solids- have ions at the points of the lattice NaCl Three types 2. Molecular solid- have discrete covalently bonded molecules at lattice points ice Three types 3. Atomic solidssubstances that have atoms at the lattice points C, B, Si and all metals Three types of atomic solids A. metallic solidsdelocalized nondirectional covalent bonding Three types of atomic solids B. network solidsatoms bond with strong directional covalent bonds that lead to giant molecules (networks) Three types of atomic solids C. Group 8 solidsnoble gas elements are attracted by London dispersion forces Structure and Bonding in Metals Metals- high thermal and electric conductivity, malleability, ductilitydue to nondirectional covalent bonding Closest packing Spherical atoms packed together and bonded in all directions spheres packed in layers-each surrounded by 6 others Three arrangements Hexagonal closest packed structure (hcp)aba arrangement has hexagonal unit cell hcp structure Every other layer has the same vertical position EX: Mg, Zn Three arrangements Cubic closest packed structure (ccp)- abc arrangement face-centered cubic cell ccp structure Every 4th level occupies the same vertical position EX: Al, Fe, Cu, Co, Ni Three arrangements Body centered cubic unit cell (bcc)spheres touch along the body diagonal of the cube bcc unit cell Most spread apart arrangement EX: alkali metals Counting atoms... Need to know number of atoms in a unit cell Face-centered 8 cubes share one cell 1/8 x 8 corners + 1/2 x 6 faces = net 4 whole spheres Density of ccp solid Ag crystallizes in a ccp structure. The radius of a silver atom is 144pm. Calculate the density of solid silver. Bonding models for metals Model must account for physical properties: A. shape can be changed fairly easilymalleable and ductile Bonding models for metals B. durable C. high melting points Bonding models for metals Indicates the bonding is STRONG and NONDIRECTIONAL or difficult to separate metals atoms, but easy to move them The model is... Electron “sea”model metal cations in sea of e (mobile e for conductivity and cations can be moved around when hammered Last topic-metal alloys! Metals introduced into the crystal structure of other metals Alloy A substance that contains a mixture of elements and has metallic properties Two types of alloys Substitutional alloysome of the host metal atoms are replaced by other metal atoms of similar size Substitutional alloys EX: Brass (1/3 of copper atoms replaced with zinc) Pewter (85%Sn, 7%Cu, 6%Bi, 2%At) Two types of alloys Interstitial alloyformed when some of the holes in a close packed metal structure are occupied by smaller atoms Interstitial alloy EX: Steel (carbon atoms into iron) Strengthens iron by adding strong directional bonds