Properties of water
advertisement

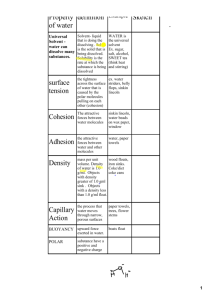
Tree Map of Water Properties Properties of Water Universal Solvent Cohesion and Adhesion Polarity Density And Buoyancy Specific Heat • Water is called the "universal solvent" because it dissolves more substances than any other liquid. – Solvent-in a solution, the liquid in which a solid dissolves – Solute-in a solution, the molecule dissolved in the solvent http://www.puritecwatersofteners.com/images/hiw_3.jpg http://ga.water.usgs.gov/edu/waterproperties.html • Cohesion-the force that holds molecules of a single material together •Adhesion-the attractive force between http://www.sci.sdsu.edu/classes/biology/bio100/truesdale/Lectures%2005/lec2/Image10.gif two different substances that are in contact with each other http://www.ccs.k12.in.us/chsBS/kons/kons/images/water-droplet.jpg • Water is polar because the oxygen has a partial negative charge and the hydrogen atoms each have a partial positive charge; polar molecules interact with other polar and charged molecules and ions O- H+ H+ • Density-the amount of the stuff/material in an object – Density = Mass/Volume • Buoyancy-Will it sink or float? – Used to determine if objects will float on the surface of water. http://www.pbs.org/wgbh/nova/lasalle/images/basics1_wood.gif • The amount of heat required to raise the temperature of 1 kg of a substance 1 degree Celsius • Water has a high specific heat index. • Water can absorb a lot of heat before it begins to get hot. http://www.scienceclarified.com/everyday/images/scet_01_img0007.jpg