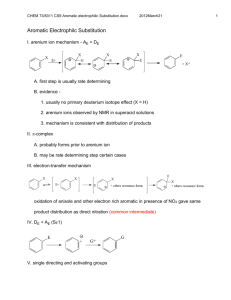
Sykes CH6 Aromatic Substitution
advertisement
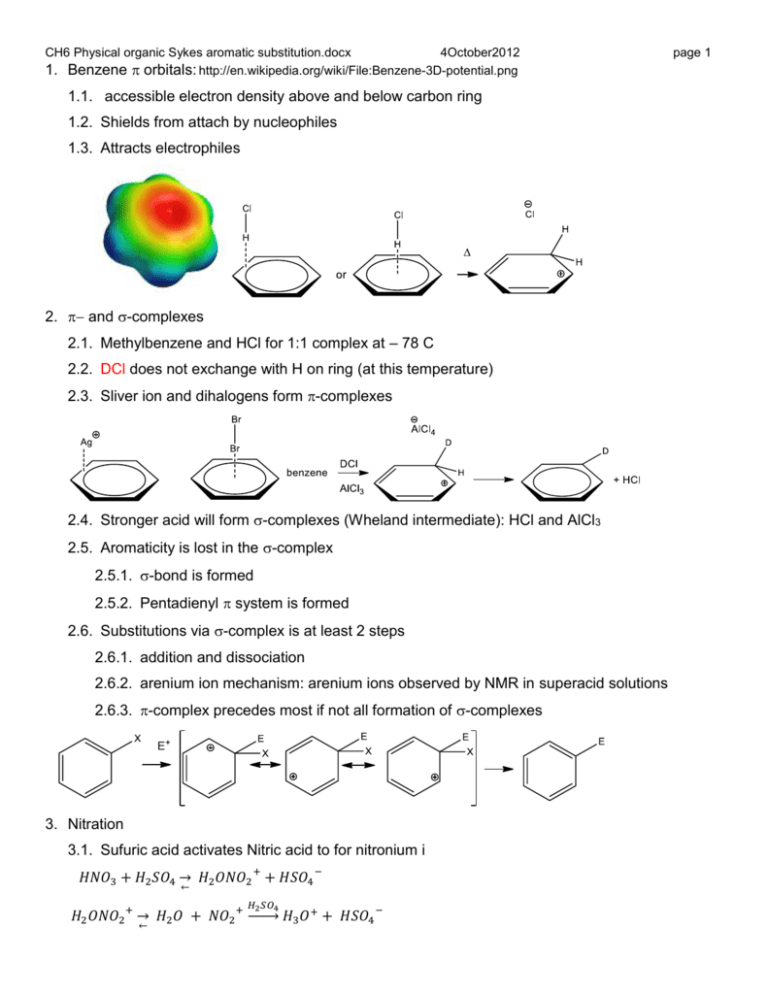
CH6 Physical organic Sykes aromatic substitution.docx 4October2012 1. Benzene orbitals: http://en.wikipedia.org/wiki/File:Benzene-3D-potential.png 1.1. accessible electron density above and below carbon ring 1.2. Shields from attach by nucleophiles 1.3. Attracts electrophiles 2. and -complexes 2.1. Methylbenzene and HCl for 1:1 complex at – 78 C 2.2. DCl does not exchange with H on ring (at this temperature) 2.3. Sliver ion and dihalogens form -complexes 2.4. Stronger acid will form -complexes (Wheland intermediate): HCl and AlCl3 2.5. Aromaticity is lost in the -complex 2.5.1. -bond is formed 2.5.2. Pentadienyl system is formed 2.6. Substitutions via -complex is at least 2 steps 2.6.1. addition and dissociation 2.6.2. arenium ion mechanism: arenium ions observed by NMR in superacid solutions 2.6.3. -complex precedes most if not all formation of -complexes 3. Nitration 3.1. Sufuric acid activates Nitric acid to for nitronium i 𝐻𝑁𝑂3 + 𝐻2 𝑆𝑂4 → 𝐻2 𝑂𝑁𝑂2 + + 𝐻𝑆𝑂4 − ← 𝐻2 𝑆𝑂4 𝐻2 𝑂𝑁𝑂2 + → 𝐻2 𝑂 + 𝑁𝑂2 + → ← 𝐻3 𝑂+ + 𝐻𝑆𝑂4 − page 1 CH6 Physical organic Sykes aromatic substitution.docx 4October2012 page 2 3.2. reaction is zero order in aromatic compound if more reactive than benzene 3.3. formation of nitronium ion is rate determining ( slow step) 3.4. Rate = k[Ar-H][NO2+] for less reactive aromatic compounds 3.5. Three mechanisms for electrophilic aromatic substitution of H 3.5.1. Difference in how electrophile attacks ring 3.5.2. There are Concerted, first step RDS, second step RDS 3.5.3. Observation: replace nitrobenzene with nitrobenzene-d5, kH/kD < 2 3.5.4. Concerted and (b) rate determining are inconsistent (CH is broken in RDS) 4. Transition state structures – Hammond Postulate 4.1. occasionally intermediates or analogs can be observed 4.2. transition state has no finite lifetime – no barrier 4.3. infer transition state structure from intermediates 4.4. “Hammond Postulate: If two states, as for example, a transition state and an unstable intermediate, occur consecutively during a reaction process and have nearly the same energy content, their interconversion will involve only a small reorganization of the molecular structures. G. S. Hammond, JACS 77 (1955) 334.” CH6 Physical organic Sykes aromatic substitution.docx 4October2012 page 3 5. Halogenation 5.1. I2 < Br2 < Cl2 (order of reactivity) occurs directly only with more reactive aromatics!!! 5.2. Benzene and unreactive aromatic require Lewis acid catalyst, e.g. AlCl3, FeX3 5.3. F2 – explosive 5.4. Rate = k[X2][arene][catalyst] 5.5. Normally No primary kinetic isotope effect – RDS is ? 5.6. Other halogenation reagents: HOCl, HOBr, HOI, ICl, CH3CO2Cl, CF3CO2Br 5.7. Note that rate laws are often complex 6. Sulfonation 6.1. Sulfur trioxide is active agent 6.2. formed in concentrated or fuming sulfuric acid 6.3. Reversible, protonation can occur before CH bond breaks (protonate water) 7. Freidel Crafts alkylation 7.1. Normally requires Lewis acid catalyst: AlCl3 >FeCl3 > BF3 > TiCl3> ZnCl2> SnCl4 (SbF5) 7.2. Rate = k[RX][arene][catalyst] or k[RX][catalyst] 7.3. Free alkyl cations form if stable, mechanism applies specifically to primary halide 7.4. Rearrangements can occur with more reactive catalyst CH6 Physical organic Sykes aromatic substitution.docx 4October2012 7.5. Exchange of halide indicates potential for rearrangement before addition to aromatic 7.6. Alkyl rearrangement also occur after product formation 7.7. Lewis acid also can cause dealkylation 7.8. Alkyl migration Hcombustion(para) = -4542 kJ/mol, Hcombustion(meta) = -4549 kJ/mol, meta is 7 kJ/mol more stable 7.9. Multiple alkylation biggest problem in synthesis – product more reactive 8. Freidel Crafts Acylation 8.1. Rate = k[RCOCl][arene][catalyst] or k[RCOCl][catalyst] 8.2. F-C acylation is more selective than alkylation 8.2.1. indicating less reactive than alkyl toward arene 8.2.2. either goes by acylium or complexed acid halide 8.2.3. acylium not detected in non-polar solvents page 4 CH6 Physical organic Sykes aromatic substitution.docx Cl Cl O + R Cl O Al Cl R Cl O Al Cl Cl Cl 4October2012 Cl Cl R Cl R H Cl Cl Al page 5 O Al C Cl Cl R O Al Cl Cl Cl R H Cl Cl Cl O Al Cl Cl Cl R Cl 8.3. benzoylchloride and benzoylbromide reaction with toluene in polar solvent 8.3.1. diferent rates but same product distribution: 1% meta, 9% ortho, 90% para 8.3.2. suggests same species attacks (PhC=O+) 8.3.3. RDS is loss of halide 8.4. In other conditions, ortho product is much lower, consistent with complex with AlX3. 8.5. More than one mole of Lewis acid required (unlike FC alkylation) 8.5.1. Ketone product a better donor than acid chloride 8.5.2. catalyst deactivated by binding to ketone 8.6. multiple acylation not a problem 8.6.1. Acylated arene is deactivated 8.6.2. Catalyst bound to acylated arene even more deactivated 8.6.3. Reduction of acyl arenes is best method to make alkyl arene 8.7. Rearrangement not a problem unless acylium can decompose to more stable ion 8.8. Other reagents 8.8.1. Anhydrides and esters can form acyliums or activated complexes , 8.8.2. Protic acids: HF, H2SO4 9. Ipso substitution 9.1. Substitutions other than hydrogen: Br, I, SiR3, SnR3, SO3H, R 9.2. Groups that attract addition (often electron rich donors) O Al Cl Cl + HCl CH6 Physical organic Sykes aromatic substitution.docx 4October2012 page 6 9.3. Desulfurization: dilute hot acid, water adds to SO3 drives reaction to completion 9.4. Silanes and stannanes (SiR3 and SnR3): Protodesilylation remove H SiR3 X- H 9.5. Protodealkylation (section 7.7 ) especially stable alkyl cations 9.6. ipso attack - five possible fates: electrophile migration, ipso group migration, ipso group loss, electrophile loss, nucleophile addition E E H Nu X X X H E -E+ -X+ X E E H X 10. Electrophilic addition to substituted benzene: directing groups 10.1. Ortho, para versus meta 10.2. Activating groups favor ortho and para 10.3. Activating (faster) or deactivating (slower) groups (relative to H, i.e. benzene) CH6 Physical organic Sykes aromatic substitution.docx 4October2012 10.4. Directing groups: meta or ortho/para, normally all three isomers form 10.5. Deactivating groups, electron withdrawing, partial or positive charge 10.6. Deactivating groups slow down attack at all positions, more at ortho and para k (C6 H 5 NMe3 ) 1.6 x10 5 for bromination (includes o, m, and p) 10.7. k (C6 H 6 ) 10.8. Most deactivating groups are meta directors (slower than benzene) 10.8.1. Avoids direct interaction with high charge at ortho and para positions page 7 CH6 Physical organic Sykes aromatic substitution.docx 4October2012 page 8 10.9. Activation by electron donating. Partial of full negative charge 10.10. Favor direct interaction with high + charge at ortho and para positions 10.10.1. o/p groups with lone pairs are inherently more stable: a fourth resonance form 10.10.2. k (C 6 H 5 OMe ) 9.7 x10 6 for chlorination (includes o, m, and p) k (C6 H 6 ) 10.11. Relative rates (repeat of 10.2) 10.12. Why is OR group activating? 10.13. Why is amide (or ester) slower then amine (or alcohol) 10.14. OMe so active mono and dibromo product reacts too fast to isolate 10.15. Anilines are deactivating in acid solution, why? CH6 Physical organic Sykes aromatic substitution.docx 4October2012 10.15.1. anilides PhNHCOR not easily protonated 10.15.2. substitute anilide and then remove acyl group 10.16. Halogens: Cl, Br, and I deactivate but are ortho and para directors 10.16.1. o/p stabilized by -donation so faster than meta 10.16.2. withdrawing effect stronger than -donation so slower than benzene 10.16.3. k (C6 H 5 Cl ) 3 x10 2 for nitration (includes o, m, and p) k (C 6 H 6 ) 10.17. The dipoles for bromobenzene and methoxybenzene are revealing 11. Direction of Multiple groups 11.1. strongest activating group controls position of substitution 11.2. position between meta groups disfavored 12. Partial rate factors – rate relative to single benzene position 12.1. f o 12.2. f m 12.3. f p k obs k benzene k obs kbenzene k obs k benzene fraction (ortho ) 6(benzene ) 2(ortho ) fraction(meta) 6(benzene) 2(meta) fraction( para ) 6(benzene) 1( para ) page 9 CH6 Physical organic Sykes aromatic substitution.docx 4October2012 page 10 12.4. for example: if nitration of trichloromethylbenzene were 15 times slower than benzene and 1 6 29% of product is para then f p 0.29 0.116 15 1 12.5. partial rate factors depend on reaction and substituent 12.5.1. does not mean that nitration < chlorination < bromination 12.5.2. doesn’t mean nitration slower than chlorination 12.5.3. nitration of benzene is faster than chlorination, and nitration of toluene is not that much faster: nitration is not as selective as chlorination 12.6. steric effect on partial rate factors: reduces ortho substitution 13. other ring systems 13.1. sulfonation of naphthalene 13.2. pyridine – more deactivated than nitrobenzene, electrophilic substitution not practical CH6 Physical organic Sykes aromatic substitution.docx 4October2012 page 11 13.2.1. : heteroatom donates only single electron to aromatic ring, N attack N E+ N N N EE H E H H 14. pyrrole 14.1. heteroatom donates a pair of electrons to aromatic ring: more reactive than benzene and directs (2) substitution (pyrrole) NH + NH H E E NH H NH NH H E H NH H NH H E E E E 14.2. dipoles indicate how different the two heterocyles are 15. Aromatic Nucleophilic Substitution 15.1. Hydride is normally not a good leaving group 15.2. Ipso nucleophilic substitution is common 15.3. Ortho/para electron withdrawing groups activate ipso substitution OMe OEt O2N NO2 OMe OEt + MeOO2N NO2 O2N 15.4. first step is usually rate determining so rate shows little dependence on L 15.4.1. rate = k[ArX][Nu] for amines and good nucleophiles 15.4.2. replacement of halogens: F > Cl > Br > I 15.4.3. first step is rate determining, F activates attack best 15.4.4. o/p withdrawing groups that can accept charge are most effective NO2 CH6 Physical organic Sykes aromatic substitution.docx 4October2012 page 12 15.5. Loss of L RDS with weaker nucleophiles like methylaniline 15.5.1. F < Cl < Br < I because loss of L is simultaneous with addition of nucleophile? 15.5.2. Unactivatived halides increase by 109 in aprotic polar solvent 15.6. 2- or 4-halopyridines not 3- readily substitute by nucleophilic substitution, what is the mechanism? 16. Aryne mechanism 16.1. Not a simple substitution 16.2. NH2- is known to exchange H and D. CH6 Physical organic Sykes aromatic substitution.docx 4October2012 page 13 16.3. Reaction is base elimination followed by addition. Orientation of products NH3 NH2 14 14 14 NH2 14 + Cl Cl NH2 14 14 NH3 NH2 14 NH2 14 + NH2 14 14 + Cl NH2 NH2 16.4. first or second step is rate determing depending on leaving group 16.4.1. proton loss is rate determining for Br and I 16.4.2. leaving group loss is rate determining for F and Cl 16.4.3. formation of most stable carbanion intermediate determines position of nucleophile attack OMe OMe NH2 Cl OMe - OMe NH2 OMe OMe - NH3 Cl NH2 OMe NH2 OMe NH2 - OMe NH3 Cl NH2 16.5. Evidence for benzyne: dimer formation during reaction and trapping Diaozocarboxylate? NH2