Electrophilic aromatic substitution: Some electrophilic aromatic substitution: H
advertisement
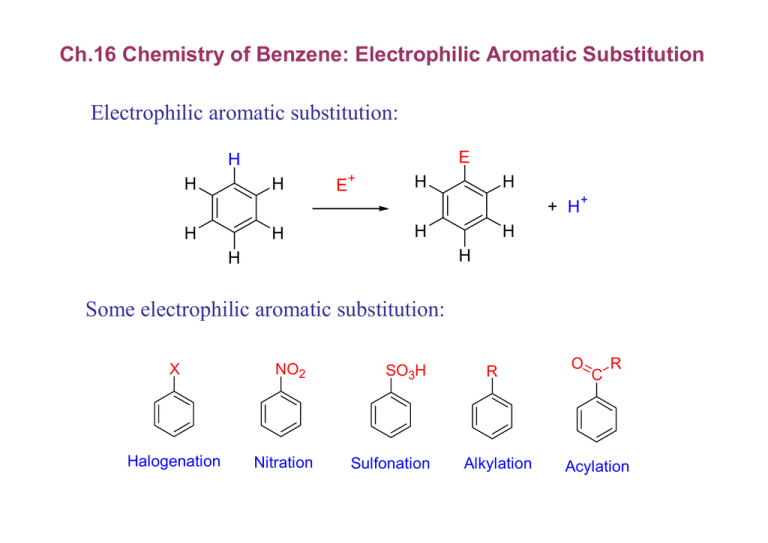
Ch.16 Chemistry of Benzene: Electrophilic Aromatic Substitution Electrophilic aromatic substitution: E H H H E+ H H + H+ H H H H H H Some electrophilic aromatic substitution: X Halogenation NO2 Nitration SO3H Sulfonation R Alkylation O C R Acylation 16.1 Bromination of Aromatic Rings Bromination: Br Br 2 + HBr FeBr 3 electrophilic addition mechanism: H Cl H H Cl- Cl - similar first electrophilic addition mechanism but aromatic rings are less reactive (more stable) than alkenes - need catalyst for aromatic electrophilic substitution FeBr 3 + Br Br δBr3Fe Br δ+ Br or + FeBr 4- + Br a strong electrophile Br + Br Br allylic carbocation three resonance forms : stable but, much less reactive than the aromatic reactant → endothermic, high Ea, slow reaction Br - electrophilic aromatic substitution need higher activation energy than alkene does E Energy E a, alkene H alkene + E + E a, benzene benzene + E + Reaction progress E overall substitution: addition + rearomatization Br+ slow Br H FeBr 4- nonaromatic intermediate Br + HBr + FeBr3 fast aromatic product X Br Br H addition nonaromatic product electrophilic bromination Energy H E Ea Br Br H benzene + Br2 Br + HBr Reaction progress 16.2 Other Aromatic Substitutions Chlorination H3C N Cl O Cl 2 + HCl cat FeCl 3 Cl N 86% Diazepam (tranquilizer) Iodination 2 I+ + 2 Cu+ l2 + 2 Cu 2+ I l2 cat CuCl 2 65% Nitration : HNO3 + H2SO4 (cat) O H+ H O NO 2+ N O N O O H O H + H 2O CH3 NO2 O2N NO2 HNO3 NO2 cat H2SO4 85% Trinitrotoluene (TNT) reduction of nitro to aniline NO2 NH2 1. SnCl2, H3O+ 2. OH- Sulfonation : fuming sulfuric acid, SO3 + H2SO4 (cat) H+ O O H S O S O O O NH2 SO3H SO3 cat H2SO4 SO2NH2 95% sulfanilimide (a sulfa drug) alkali fusion reaction SO3H OH 1. NaOH, 300oC 2. H3O CH3 p-Cresol (72%) + CH3 16.3 Alkylation of Aromatic Rings: The Friedel-Craft Reactions Friedel-Craft Alkylation Cl AlCl 3 + H 3C CH 3 H 3C CH 3 + AlCl 4- Cl AlCl 3 + H 3C CH 3 + HCl Cumene (85%) Limitation: - only useful for RCl not for ArCl or vinyl chloride - aromatic rings with electron-withdrawing groups are unreactive - aromatic rings with amino group are unreactive: amines under go acid-base reaction with AlCl3 Y + AlCl 3 R X NO reaction Y = NR3+, NO2, CN, SO3H, CHO, COR, COOH, COOR = NH 2, NHR, NR 2 polyalkylation problem C(CH 3)3 + (CH 3)3CCl AlCl 3 C(CH 3)3 + C(CH 3)3 minor - use excess of benzene for mono alkylation major skeleton rearrangement: carbocation + AlCl 3 + Cl 65% 35% carbocations: skeleton rearrangement to a more stable cation CH 2 H Hydride shift H H 3C CH 2 H 3C CH 3 + Alkyl shift H 3C CH 3 CH 3 Cl AlCl 3 16.4 Acylation of Aromatic Rings Friedel-Craft Acylation CH 3 O O AlCl 3 + Cl CH 3 + HCl 80oC Acetophenone (95%) O AlCl 3 + Cl R O C + R O C R - no polyacylation: less reactive acyl product AlCl 4- 16.5 Substituent Effects in Substituted Aromatic Rings 1. Reactivity NO 2 relative rate of nitration 6 x 10-8 Cl 0.033 H 1 OH 1000 2. Orientation OH OH OH OH NO 2 HNO 3 + + H2SO4, 25oC NO 2 o-Nitrophenol 50% CN CN m- NO 2 p- 0% 50% CN CN NO 2 HNO 3 + + H2SO4, 25oC NO 2 o-Nitrobenzonitrile 17% m- NO 2 p- 81% 2% Substituent Effects in Electrophilic Aromatic Substitution electron-poor -SO3H -NO2 -NR3+ -CN -COOH electron-rich -CHO -COCH3 -COOCH3 meta-directing deactivators -I -Ph -F -Br -Cl ortho- and paradirecting deactivators no meta-directing activators -H -OMe -OH -CH3 -NHCOCH3 alkyl ortho- and paradirecting activators -NH2 Two factors in activating/deactivating effect: inductive effect: electronegativity differences resonance effect: lone pair electrons, double bond Inductively withdrawing groups - positive charges at the neighboring atom inductively withdrawing − δ + δ X δ−O C δ+ R δ−O C δ+ OR C δ+ X= F, Cl, Br, I inductively donating R δ− N O N − δ+ O δ Resonance effect: withdraw or donate electrons through a π-bond - the effect is greatest at the ortho and para positions electron withdrawing resonance effect O C O C R δ− Z Y+ δ O C R δ−O C δ+ R C δ+ δ− N O C R O N − δ+ O δ R Electron donating resonance effect - the effect is greatest at the ortho and para positions OH Y O O H NH 2 OH O H OR H X X= halogen - inductive effect and resonance effect don't necessary act in the same direction ; -X, -O, -N atoms are inductively withdrawing groups but electrondonating resonance effect 16.6 An Explanation of Substituent Effects Activating/deactivating effect Activating groups: donate electrons to the ring - stabilize carbocation intermediate - lower the activation energy for carbocation formation Deactivating groups: withdraw electrons from the ring - destabilize carbocation intermediate - raise the activation energy for carbocation formation Y > E+ Y H > E+ E+ Y H Y E stabilized carbocation E E destabilized carbocation Orientating Effect ortho, para directors: - lone pair electrons - stabilize carbocation intermediate by resonance NH 2 OH R halogens: - inductively deactivating - but ortho, para directing by resonance stabilization Br alkyl group: ortho, para activator CH 3 NO 2 NO 2+ ortho 63% CH 3 NO 2 NO 2 Most stable CH 3 CH 3 CH 3 meta 3% CH 3 NO 2 CH 3 NO 2 NO 2 CH 3 CH 3 CH 3 NO 2 NO 2 NO 2 para 34% Most stable OH, NH2 group: ortho, para activator OH OH NO 2 NO 2+ ortho 50% NO 2 OH NO 2 NO 2 Most stable OH OH OH meta 0% OH NO 2 OH NO 2 NO 2 OH OH OH OH NO 2 NO 2 NO 2 NO 2 para 50% Most stable - stabilizing resonance interactions for ortho and para additions halogen group: ortho, para adectivator Cl Cl NO 2 NO 2+ ortho 35% NO 2 Cl NO 2 NO 2 Most stable Cl Cl Cl meta 1% Cl NO 2 Cl NO 2 NO 2 Cl Cl Cl Cl NO 2 NO 2 NO 2 NO 2 para 64% Most stable - inductively deactivating - stabilizing resonance interactions for ortho and para additions EWG group: meta deactivator δ+CHO Cl + CHO Cl CHO Cl Cl ortho δ− O 19% H C δ+ Least stable CHO meta CHO Cl 72% CHO Cl Cl CHO CHO CHO Cl Cl Cl para 9% Least stable destabilizing inductive interactions for ortho and para additions A Summary of Substituent Effects in Aromatic Substitution Substituents Reactivity Orientation Inductive Effect Resonance Effect -CH3 activating ortho para weak; electrondonating none -OH, -NH2 activating ortho para weak; electronwithdrawing strong; electrondonating -F, Cl, Br, I deactivating ortho para strong; electronwithdrawing weak; electrondonating -N+(CH3)3 deactivating meta strong; electronwithdrawing none -NO2, -CN, CHO, -CO2Me, - deactivating COCH3, -COOH meta strong; electronwithdrawing strong; electronwithdrawing 16.7 Trisubstituted Benzenes: Additivity of Effects 1. two groups reinforce each other: CH 3 CH 3 NO 2 HNO 3 H2SO 4 NO 2 NO 2 2. two groups oppose each other: more powerful directing group wins, but mixture of products often result CH 3 CH 3 Br 2 Br OH OH 3. sterically hindered site: further substitution rarely occurs between the two groups in a metadisubstituted compound too hindered CH 3 Cl 2 Cl CH 3 CH 3 CH 3 Cl Cl + FeCl 3 Cl Cl Cl Cl NOT formed - alternative preparation 1,2,3-trisubstituted compound CH 3 CH 3 NO 2 HNO 3 O 2N CH 3 NO 2 NO 2 + H2SO 4 NO 2 16.8 Nucleophilic Aromatic Substitution nucleophilic aromatic substitution: no SN1, SN 2 mechanism OH Cl O 2N NO 2 1. NaOH O 2N NO 2 + Cl- 2. H3O+ NO 2 NO 2 100% sp2 orbital (unstable) Cl + Cl- X no SN1 reaction HO Cl X no SN2 reaction addition/elimination mechanism Cl NO 2 - OH Cl OH OH NO 2 + Cl- NO 2 Meisenheimer complex - nucleophilic aromatic substitution occurs only if the aromatic ring has electron withdrawing group(s) in ortho or para position to the halogen - meta substituent has no resonance stabilization nucleophilic aromatic substitution: need ortho or para EWG Cl - OH NO 2 NO 2 - para Cl OH O 2N OH O 2N Cl meta - OH OH O N O Cl OH X NO 2 OH O N O Cl OH ortho Cl Cl Cl NO 2 no stabilization of charge by nitro group 16.9 Benzyne nucleophilic aromatic substitution of non-activated system ; need high temperature and high pressure OH Cl 1. NaOH, H 2O, 340 oC, 2500psi + NaCl 2. H3O+ benzyne intermediate; elimination/addition mechanism OH Cl H - OH H 2O - HCl elimination addition Benzyne H evidence for benzyne mechanism: 14C labeling at C1 Cl * H KNH 2 NH 3 * NH 3 * NH 3 addition Benzyne symmetrical 50% H H + * 50% NH 3 trapping benzyne intermediate O Br H KNH 2 O NH 3 Diels-Alder adduct Benzyne dienophile H C C H C H C H C C 16.10 Oxidation of Aromatic Compounds Oxidation of Alkylbenzene Side Chains aromatic rings; inert to KMnO4 benzylic CH2: oxidized to -COOH by KMnO4, Na2Cr2O7 KMnO 4 COOH H 2O industrial procedure COOH CH 3 O2 Co (III) CH 3 COOH CH 3 attack benzylic C-H bonds CH 3 CH 3 H 3C C KMnO 4 H 2O NO reaction Bromination of Alkylbenzene Side Chains Br O NBS (PhCO 2)2 CCl 4 + N H O radical mechanism H H R H Br H R Br 2 R + HBr O HBr + N Br O O Br2 + Br N H O Br resonance stabilized benzylic radical H H H H H H H H 16.11 Reduction of Aromatic Compounds Catalytic Hydrogenation of Aromatic Rings aromatic rings; inert to normal hydrogenation conditions O O H2, Pd EtOH but, at high pressure of H2 and high temperature or use reactive rhodium catalyst ; reduced to cycloalkanes CH 3 CH 3 Pt EtOH o 25 C HO CH 3 H2 (2000 psi) H2(1 atm) Rh/C EtOH 25oC 100% CH 3 HO 100% Reduction of Aryl Alkyl Ketones ; neighboring carbonyl groups are reactive under reducing condition O O H2, Pd Cl AlCl 3 EtOH AlCl 3 + Cl nitro groups are reduced under the reaction conditions O O 2N H2, Pd EtOH H 2N synthesis of complex molecules starting from simple precursors; - pharmaceutical industry: new drugs - chemical industry: economical routes to known compounds - academic: applications + pure challenges planning synthesis needs - knowledge about organic reactions - practical ability: any problems retrosynthetic analysis: design reaction schemes backward in case complex molecules 16.12 Synthesis of Trisubstituted Benzenes NO 2 ? ..... Cl HNO 3 H2SO 4 NO 2 NO 2 Cl 2, FeCl 3 X Cl HNO 3 H2SO 4 Cl Br ? COOH Br Br KMnO 4 COOH Br CH 3Cl AlCl 3 Br2, FeBr 3 CH 3 Br 2, FeBr 3 Br 2, FeBr 3 CH 3Cl, AlCl 3 COOH CH 3 O O Cl H2, Pd/C Cl Cl NO 2 deactivated ring will not undergo Friedel-Craft rxn NO 2 HNO 3 H2SO 4 ;Cl NO 2 no correct isomer Chemistry @ Work Combinatorial Chemistry R4 O N R3 R1 N R2 + + Benzodiazepine library (R1-R4 are various substituents) Chemistry @ Work 2,180,106 compounds Problem Sets Chapter 16 28, 33, 35, 40, 54, 64, 70