Gas Unit and Pressure

Gas Laws
• The Gas Laws
• Kinetic Molecular Theory
• .
Matter is composed of very tiny particles
• .
Particles of matter are in continual motion
• .
The total KE of colliding particles remains constant
- When individual particles collide, some lose energy while others gain energy.
- No overall energy loss = elastic collisons
Properties of a Gas
• Expansion – no definite shape or volume
• Low density – density of gas is about 1/1000 of that same a solid substance in a liquid or phase
• Diffusion – process of spreading out spontaneously to occupy a space uniformly
Ideal Gas
• consists of very small independent particles. These particles move at random in space and experience elastic collisions
• Extra – Approximate speed is 10 3 meters/sec, at 0 degrees C and 1 ATM of pressure
• Undergo: 5 x 10 9 collisions/second
Gases in Real life are not so ideal
At short distances the nucleus of one atom is attracted b y the negat ively charged e lectron orbitals of another a tom. It is these forces that cause gas molecules to come togethe r and condense to a liquid when the speeds o f the gas molecules are slowed enough to allow the attractive forces to ope rate.
When would a gas not act
“ideal”?
• At low temperatures - the molecules may be moving slow enough to allow attractions to occur
• At high pressures - the molecules may once again have attractive forces between them in effect
and
Gases
Pressure
Pressure – the total average force over a given area is called pressure . Although the collisions of any single molecule of gas within the walls of a container are intermittent, there are so many molecules in even a small container, that the tremendous number of collisions average out to a steady pressure.
Gas and Temperature
• Temperature – the average kinetic energy or motion energy of its molecules.
• Avogadro’s hypothesis states that at any given temperature and pressure, equal volumes of gases, contain the same number of molecules.
• The following table supports the hypothesis by showing that one mole (6.02 x 10 23 molecules) of 11 different gases occupies approximately the same volume. These volumes were derived experimentally at the same pressure and temperature.
Table 1. Volumes of various gases at 0 C and 760 mm pressure
1 mole: Oxygen
Sulfur Dioxide
Hydrogen
Helium
Chlorine
Nitrogen
Ammonia
Hydrogen Chloride
Carbon Monoxide
Carbon Dioxide
Occupies: 22.393 L
Occupies: 21.888 L
Occupies: 22.430 L
Occupies: 22.426 L
Occupies: 22.063 L
Occupies: 22.403 L
Occupies: 22.094 L
Occupies: 22.248 L
Occupies: 22.402 L
Occupies: 22.262 L
Why should one mole of hydrogen occupy the same volume as one mole of oxygen?
Since oxygen molecules are about
16 times as heavy as hydrogen molecules, it would seem more reasonable for a mole of oxygen molecules to occupy much more volume than a mole of hydrogen molecules at the same temperature and pressure.
Remember the Demo?
• The smaller balls make up in speed what they lack in size in “carving out” an area.
This reasoning can be applied to the volume occupied by lighter gas molecules compared to the identical volume occupied by an equal number of heavier gas molecules at the same temperature and pressure.
Van der Waal Forces
• London Dispersion Forces - (Van der Waal’s Forces)
– the attractive forces between gas molecules.
• Even though the total electrical charge of the gas molecule is neutral, the positively charged nuclei of its atoms are not completely shielded by negatively charged electrons.
Van der Waal’s
In general, the magnitude of
Van Der Waal’s forces increases with an increase in the number of electrons per atom and with an increase in size of the molecule.
Few electrons = lame wave
Many electrons = more powerful wave!!
STP conditions
• Standard temperature and pressure – useful when wanting to compare or measure gas volumes
• ST = 0 C = 273K
• SP = 760mm of Hg = 1atm = 760 Torr
• STP = standard temperature and pressure
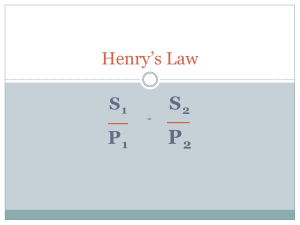
Boyle’s Law
• Boyle’s Law – the volume of a definite quantity of dry gas is inversely proportional to the pressure, provided temperature remains constant.
(V 1/P)
• Formulas or dimensional analysis?
P
1
V
1
= P
2
V
2
Boyle’s Law,
Continues
Examples:
• Heimlich maneuver (increase P in lungs by decreasing their V)
• Syringe
• Straw (Lungs are acting as the chamber that increases in V)
• V P & V P
Problem 1
Suppose a syringe plunger is pushed all the way in so that the syringe contains
0.10 mL of air at 1.00 ATM. The plunger is now pulled back quickly so that the total volume changes to 3.00 mL.
Calculate the momentary pressure before any liquid comes into the syringe.
Solve using dimensional analysis
Initial P is 1.00 ATM so you have two possible conversion factors:
A. 0.10 mL
----------
3.00 mL
OR B. 3.00 mL
----------
0.10 mL
Which do we choose?
Think: If we are increasing V we expect the gas P to decrease so we want the conversion factor that is less than one.
1.00 atm x 0.10 mL = 0.033 atm
----------
3.00 mL
The plug-and-chug (no thinking involved) method
P
1
V
1
= P
2
V
2
1.00 atm x 0.10 mL = P
2 x 3.00 mL
P
2 =
0.033 atm
Problem 2
A small child carrying an inflated balloon with a volume of 2.00 liters gets on an airplane in N.Y. at a P of
760. Torr. What will the volume of his balloon be on arrival at Mexico City, which is 7347 feet above sea level and has an average P of
600. Torr?
Answer to problem 2
2.00 L x 760. Torr
----------= 2.53 Liters
600. Torr
Solve, p&c method #2 = P
1
V
1
= P
2
V
2
760. Torr x 2.00 Liter = V
2 x 600. Torr
V
2
= 2.53 Liters
Charle’s Law
• The volume of a gas is directly proportional to the Kelvin Temperature (Pressure remains a constant).
V T
V
1
= V
2
T
1
T
2
Problem 1
The initial volume of a piston is 1.5 Liters at 300.K.
What will be the final volume at 600. K? (P is constant)
– Think: Since volume temperature, as V increases then T must increase. So, use the conversion factor that is greater than 1
Answer
1.5 Liter x 600.K = 3.0L
300.K
Or solve way #2 = V
1
/T
1
V
2
= V
2
1.5L / 300.K = V
2
/ T
2
/600.K
= 3.0L
Problem 2: Suppose a balloon had a volume of 2 x 10 5 liter when it was filled with hot air at a temp. of 500. C. What volume would it occupy if it were sealed and cooled at 27 C?
Answer to Problem 2
Think: If T decreases the V would also decrease. So, choose a factor less than 1.
Solve: 500. C = 773 K AND 27 C = 300. K
( 2 x 10 5 L ) x 300.K
= 8 x 10 4 L
773K
Gay-Lussac’s Law
The pressure inside a fixed volume is directly proportional to the
Kelvin temperature.
P T
Example: Spray can warning:
“Do not incinerate!”
P
1 =
P
T
1
T
2
2
Gay-Lussac’s, continued
The contents of a spray can are all used up and the can thus contains gas at a pressure of 1.00 atm.
The can is thrown into a fire where it’s T rises to
627 C. Why does the can explode? -- Started at a temperature of 27 C.
– Think: If T increases, the P must increase if there is no change in volume. So, solve for pressure .
Solving for this Law
• The pressure inside the can, right before it blew up, would have been:
• Remember if T , P
1.00 atm x 900. K = 3.00 atm
300. K
Problem 2
Problem: A pressure cooker is closed and is at 20. C. To what temperature must the gas be heated in order to create an internal pressure of 2.00 atm?
Solving problem 2
Solve: P1 = 1.00 atm and it has to increase to
2.00 atm. So, P needs to increase and that means temperature must also increase.
293 K x 2.00 atm
1.00 atm = 586 K
Combining the gas laws
• P
1
V
1
= P
2
V
2
T
1
T
2
Whoops, wrong combining.
Combination problem
• Suppose you have a balloon that has a volume of 20.0 L at a pressure of 1.00 atm and a temperature of 32.0 C. If this balloon rose into the atmosphere where the temperature was only 12.0 C and the pressure only 0.82 atm, what would its new volume be?
First convert the temperatures to Kelvin:
12.0 + 273 = 285 K
32.0 + 273 = 305 K
Second: either plug in values into your combination formula or do the THINKING method
P so
V
&
T so
V
• 20.0 L x 1.00 atm x 285 K = 23 L
0.82 atm 305 K
Dalton’s Law of Partial Pressure
• the total pressure of the mixture of gases is the sum of their partial pressures
• P
TOTAL
= P
A
+ P
B
+ P
C
+ ….ETC
Problem 1
A mixture of gases in a 3.00 L flask consists of
Nitrogen at a partial pressure of 100 Torr and
Oxygen at a PP of 300 Torr. What is the volume of each gas? Calculate the total pressure in the flask.
Since the presence of another gas has no effect on the volume available to the first gas, both
Nitrogen and Oxygen have a volume of 3.00L
Ans. P
TOTAL
= 100 Torr + 300 Torr = 400 Torr
Problem 2
Air has a relative constant proportion of N
2
(78.10%), O
2
(21.00%), Ar (0.90%), and CO
(0.03%). The partial pressure of each gas in
2 normal dry air at sea level is proportional to the number of molecules. What is the Total atmospheric pressure?
N
2
= 0.7810 atm. + O
2
Ar = 0.0090 atm. + CO
2
= 0.2100 atm. +
= 0.0003 atm. = answer. P
TOTAL
= 1.0003 atm
Graham’s Law of Diffusion
• Diffusion rate depends on 3 factors:
– Speed of the molecules (the higher the temperature, the higher the average KE)
– Diameter of the molecule (the larger the molecule, the slower the diffusion rate)
– Potential Attractive forces (the greater the potential attractive forces between the molecules, the slower the diffusion rate)
Graham’s Law
• For an average molecule - A and an average molecule - B, use the following formula to determine velocity:
V
A
= M
B
V
B
M
A
Graham’s Law Sample Problem
• At room temperature, an average hydrogen molecule travels at a speed of
1700. meters/second (about 3000 miles/hr). What is the speed of an average oxygen molecule under the same conditions?
Solving for Sample Problem 1
1700. m/sec = 32.0 amu
V
B
2.02 amu
So, V
B
= 425 m/sec
Another awesome formula
• Problem: A steam autoclave is used to sterilize hospital instruments. Suppose an autoclave with a volume of 15.0 liters contains pure steam (water) at a temperature of 121 C and a P of 1550 Torr. How many grams of water does it contain?
• Solve: PV = nRT
• R = 62.4 L * Torr / Mole * K
Solving using the Ideal Gas Law
• 1550 Torr x 15.0 L = n x 62.4 L * Torr x 394 K
Mol * K
• n = 0.946 moles of H
2
O
• 0946 moles of H
2
O x 18.0g H
2
O = 17.0 g H
2
O
1 mole H
2
O
• NOTE: You could solve using your knowledge of combination gas laws and molar volume
(at STP)
Another Sample Problem
• Example 2: A hospital uses an oxygen gas cylinder containing 3.50 kg of O
2 gas in a volume of 20.0 L and at a T of 24 C. What is the P in atm in the cylinder?
Problem Solved
PV = nRT n = 3500 g O
2 x 1 mole/32.0 g = 109 moles
P( 20.0 L) = (109 moles)(0.0821 L x atm/mole x K)(297 K)
P = 133 atm
Eudiometer Tubes
(mercury or water displacement)
• Eudiometer – a tube used to collect a gas.
It is closed at one end and is graduated.
• Reaction = 2HCl (aq) + Ca (s) H
2
CaCl
2
(g) +
(aq)
Eudiometer with mercury
Situation #1
• If there is just enough gas to make the mercury level inside the tube the same as the level of the mercury in the bowl then the pressure of the hydrogen gas is the same as that of the atmosphere!
Eudiometer, Situation #2
• Suppose enough hydrogen gas is added to make the level inside the tube lower than the level of the mercury in the bowl. The P of the hydrogen gas is greater than the atmospheric pressure. (To determine the hydrogen gas pressure one must add the level difference to the barometric reading.)
• Problem situation 2: The volume of oxygen in a eudiometer is 37.0 mL. The mercury level inside the tube is 25.00mm lower than the outside. The barometric reading is 742.0 mm Hg.
Solving for Problem 2
Solution 2: 742.0mm + 25.00mm = 767.0mm
Eudiometer with mercury, situation #3
Suppose there was not enough gas to make the mercury level the same. Then, the pressure of hydrogen gas would not be the same as the pressure of the air outside the tube. (So, we must subtract the level difference from the barometric reading.)
• Problem situation 3: What is the P of the gas in an eudiometer, when the mercury level in the tube is 14mm higher, than that outside? That barometer reads 735mm Hg.
Answer to Problem 3
• Solution: 735mm – 14mm = 721mm
Water in Place of Mercury
• Water is often used in place of mercury.
Calculations are done the same way but the difference in water levels must first be divided by 13.6
to convert it to its equivalent height in terms of a column of mercury since water is 1/13.6 as dense as mercury.
New Problem: If you collected some oxygen gas in the eudiometer by the method known as water displacement you must:
• Correct for the difference in density between water and mercury!!!
• Also, water evaporates much more readily than mercury and so is in with the collected gas. So, you need to determine the partial pressure of the dry gas (unmixed with water vapor). The vapor pressure of water, at the given temperature, must be subtracted from the total pressure of the gas within the tube.
• Problem 1: Oxygen is collected using the water displacement method. The water level inside the tube is 27.2mm higher than that outside. The temp. is 25.0 C. The barometric pressure is 741.0mm. What is the partial pressure of the dry oxygen in the eudiometer?
Step 1: 27.2mm/13.6 = 2.00mm
Step 2: 741.0 mm - 2.00mm = 739.0 mm
So: 739.0 mm = P total = Poxygen + Pwater
Step 3: To correct for water vapor pressure, you need to know the pressure of water vapor at 25.0C and it is
23.8mm (always given - see handout). Subtract this water partial pressure from the pressure total found in step 2.
739.0mm - 23.8mm = 715.2 mm = partial pressure of the dry oxygen.
• Problem 2: A eudiometer contains 38.4 mL of air collected by water displacement at a temperature of 20.0C. The water level inside the eudiometer is 140. mm higher than that outside. The barometric reading is
740.0mm. Water vapor pressure at 20.0C is
17.5mm. Calculate the volume of dry air at
STP.
•First Step: 140mm/13.6= 10.3 mm
•Second Step: 740.0mm - 10.3mm = 729.7mm
• Third Step: 729.7m
m - 17.5mm = 712.2 mm
pressure at a Temp. of
293K
• Now it asks you to go a step further and calculate what the volume would be at standard temperature and pressure which = 760. mm and 273K. To solve this problem you need to combine two gas laws –
Charles and Boyles. Think of your possible conversion factors and solve.
• 38.4mL x ________ x __________ =
Combination finish
• 38.4mL x 273 K x 712.2mm = 33.5 mL
293 K 760. mm
More chemistry problems involving gases:
1. Gas volume – gas volume
2. Mass-gas volume
Avagadro’s principle: Equal volumes of all gases, under the same conditions of T and P contain the same number of molecules.
Further, the number of molecules (of all gases) are in the same ratio as their respective gas volumes. (Refer back to molar volume definition)
Gay Lussac’s law
• Under the same conditions of T and P, the volumes of reacting gases and their gaseous products are expressed in ratios of small whole numbers.
• 2H
2
(g) + O
2
(g) --> 2H
2
O(g)
2 volumes of H
2
+ 1 vol. of O
2
2 vol. of H
2
O vapor.
Sample problem 1
• What is the volume of oxygen that would combine with 4.0 liters of hydrogen to form water vapor?
• Equation =
2H
2
(g) + O
2
(g) 2 H
2
O
Answer: 4.0 L H
2 x 1 vol. O
2 =
2.0 L of O
2
2 vol. H
2
Sample Problem 2
• What is the volume of hydrogen chloride gas that would be produced, assuming
5.0 liters of chlorine gas fully reacted with hydrogen gas?
Equation:
H
2
(g) + Cl
2
(g) --> 2HCl(g)
Answer: 5.0 L Cl
2 x 2 vol. HCl =
1 vol. Cl
2
10.0 L of HCl
Remember Molar Volume?
• How many grams of CaCO
3 must be decomposed to produce 4.00 liters of CO
2
STP? (Calcium oxide is also formed) at
• CaCO
3
(s) CaO (s) + CO
2
(g)
• 1 mole--------------------1mole (stoich. ratio)
• 1 mole CaCO
3
= 100.1g
• 1 mole CO
2 at STP
= 22.4 liters (remember?)
Molar volume revisited
(or ideal?)
• 4.00 L x 1 mole/22.4 L = 0.179 moles CO
2
• Since CO
2 and CaCO
3 are on a 1/1 mole ratio, you will need 0.179 moles of CaCO
3
• 0.179 moles CaCO
3 x 100.1 g CaCO
3 =
17.9 g
1 mole CaCO
3