Chapter 6 The States of Matter
advertisement

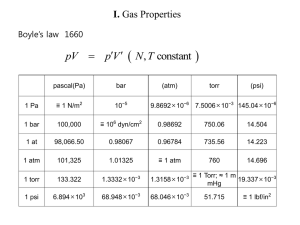
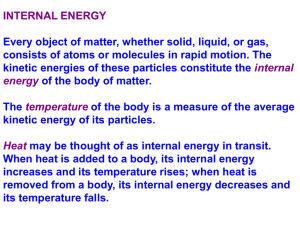
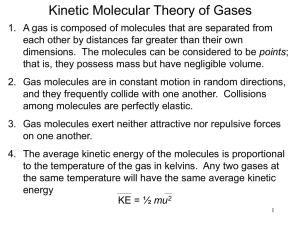
Chapter 6: The States of Matter Suggested Problems: States of Matter • ______ – Definite volume and shape • ______ – Definite volume but not shape • Takes the shape of its container • _______ – No definite volume or shape • Will not only take the shape of its container it will fill it completely Solids • A __________ state of matter • Atoms (or molecules) are “touching” • Strongest intermolecular forces – Hold atoms (or molecules or ions, etc.) rigidly in a 3D crystalline lattice Liquids • Also a __________ state of matter • Atoms (or molecules) are “touching” • Intermolecular forces hold atoms (or molecules) in contact, but not rigidly in place…molecules can slide past each other Gases • Virtually ___ intermolecular forces • Gaseous molecules (etc.) comprise a very small percent of the sample volume • Gaseous molecules are in constant random motion…the velocity is related to temperature • Molecules collide with walls of container and with each other and bounce off with no loss of energy Properties of Gases • Gases are the best understood state of matter, because we ignore intermolecular forces • The volume a gas sample occupies is a function of three variables: – ___________ – ___________ – ___________ Pressure • Pressure is force per unit area • Units of pressure: – Pounds per square inch – Torr or mmHg – Atmosphere 1 atm = 760 torr = 760 mmHg = 14.7 psi Measuring Pressure: Barometer gravity pulling down air pushing down Pressure Conversion Example • The gauge on an oxygen gas cylinder reads 1272 psi. Express this in atm and torr. (1 atm=14.7 psi) Volume of a Gas • Imagine a fixed amount of air at a given temperature and pressure in a balloon • What will happen to the volume if we add more air? Volume of a Gas • Imagine a fixed amount of air at a given temperature and pressure in a balloon • What will happen to the volume if we squeeze the balloon (increase pressure)? Volume of a Gas • Imagine a fixed amount of air at a given temperature and pressure in a balloon • What will happen to the volume if we increase the temperature? The Combined Gas Law P1V1 P2 V2 n1T1 n 2 T2 • P is pressure • V is volume • T is ________ temperature • n is number of moles The Empirical Gas Laws • Boyle’s Law – Volume is inversely proportional to pressure (constant n and T) • Charles’s Law – Volume is directly proportional to the Kelvin temperature (constant n and P) • Avogadro’s Law – The volume of a gas is directly proportional to the number of gas moles (constant T and P) Boyle’s Law: Example • 15 liters of argon is collected at an initial pressure of 1.05 atm. If the gas is compressed to a new pressure of 1510 torr, what is the new volume? Charles’s Law: Example • The temperature of 35.6 mL of neon is increased from –35.4ºC to 75.2ºC. What is the new volume? Combining Boyle’s and Charles's Laws • A bubble of air having a volume of 75.0 mL is released from 35 feet under the sea (where the pressure is 2.07 atm and the temperature is 18 ºC). What will the new volume be at the surface, where P=0.967 atm and T=23 ºC? The Ideal Gas Law • Combines the elements of the ________ gas laws P1V1 P2 V2 constant R n1T1 n 2T2 P V nRT L atm R 0.0821 mol K Standard Conditions • STP = standard temperature and pressure T = _____ K (_____ ºC) P = _____ atm = ____ torr Example • What volume will 2.0 grams of helium occupy at a temperature of 290K and a pressure of 800 torr? Which Gas Law to Use? • Use the combined gas law when the problem describes two sets of conditions – the pressure and/or temperature changes • Use the ideal gas law when there are a single set of conditions Dalton’s Law of Partial Pressures • The ______ pressure of a gaseous mixture is the sum of the partial pressures Nitrogen 0.75 atm Nitrogen 0.75 atm Empty container Oxygen 0.25 atm Ptotal = Oxygen 0.25 atm Graham’s Law • __________ is net movement of a gas from an area of high concentration (pressure) to an area of lower concentration • __________ is the movement of a gas through a pinhole • Both Diffusion and Effusion follow Graham’s Law Rate1 MW2 Rate2 MW1 Rate is an amount per time Graham’s Law Example • Oxygen Molecules weigh 16 times as much as hydrogen molecules. Which molecule will diffuse faster and how much faster? Changes of State Gas freeze Liquid melt What do all of these changes in state have in common? Solid Energy • Energy is the ability to do work – Kinetic Energy: energy due to motion – _________ Energy: stored energy – Heat Energy: the sum of the kinetic and potential energies of molecules in a sample Energy and Its Units • calorie (cal): is the amount of heat needed to raise the temperature of 1 gram of water o by 1 degree Celsius at 15 C • kilocalorie = 1000 cal • A food Calorie = 1000 cal • Joules (J): are the metric unit of energy 1 cal = 4.184 J Energy Conversion Example • A candy bar has 350 Calories. How many joules does one candy bar contain? Heat and Temperature • __________: is a measurement of the average kinetic energy of the molecules in a sample – ___ is measured in degrees with a thermometer • _______: is the sum of the kinetic (and potential) energies in a sample – _____ is measured in calories with a calorimeter Calorimeters Bomb Calorimeter Coffee Cup Calorimeter Specific Heat • Specific heat (SH) is the amount of heat needed to raise the temperature of 1 gram of material by one degree Celsius q (mass)(SH)(T ) q SH mass T q heat T (TF TI ) Specific Heat Example • A 10.0 gram sample of copper at 25 ºC has a final temperature of 100 ºC when 289 J of heat are added. What is the specific heat of copper? (SH of liq water = o 4.18 J/g C) Distribution of Energy • In a sample of material, the kinetic energies of the molecules follow a Boltzman Distribution: # Molecules Average KE 1 KE mv2 2 m mass v velocit y Kinetic Energy Kinetic Energy Distribution # Molecules Tlow Thigh Kinetic Energy Changes of State Gas freeze Liquid melt Solid Vaporization • The most energetic molecules in a liquid have sufficient kinetic energy to escape into the ____ phase • Once the molecules are free as gases, they exert a pressure – This is called the ______ pressure • How does vapor pressure depend on temperature? Vapor Pressure of Water and Ethanol 1000 900 800 Vapor Pressure (Torr) 700 vapor pressure of ethanol 600 500 400 vapor pressure of water 300 200 100 0 0 20 40 60 Temperature ( oCelcius) 80 100 Boiling Point • The boiling point of a liquid is the temperature where the vapor pressure equals the ambient pressure. • The _______ boiling point of a liquid is the temperature where the vapor pressure equals 760 torr. • How does boiling point depend on pressure? Changes of State Gas freeze Liquid melt What do all of these changes in state have in common? Solid Freezing/Melting Point • The melting point of a substance is the temperature at which a crystalline solid changes to a liquid. • What is the difference between melting point and freezing point? Energy Changes and Changes of State • Imagine recording the temperature of an 18 gram (i.e., 1.0 mole) sample of ice at -40ºC as heat is added heat added, kJ 0.0 1.5 7.5 15.0 55.7 56.5 temperature -40 0 0 100 100 120 No T No T Heating Curve for 1 Mole of Water 120 100 Water is boiling: Heat of vaporization 40.7 kJ/mol Temp (oC) 80 60 40 20 Ice is melting: Heat of fusion 6.02 kJ/mol 0 -20 -40 0 10 20 30 kilojoules of heat added 40 50 60 Molar Heat of Fusion Hºfus is the heat required to convert one mole of solid to a liquid at at its normal melting point Hºfus represents the energy needed to break down intermolecular forces and allow molecules to slide around the liquid phase Molar Heat of Vaporization H°vap is the heat required to convert one mole of liquid to a gas at at its normal boiling point H°vap represents the energy needed to break intermolecular forces and allow molecules to escape into the gas phase Putting it all Together • How much heat is required to convert an 18 gram piece of ice at -40 oC to steam at 120 oC? Heating Curve for 1 Mole of Water 120 D 100 Heat of vaporization 40.7 kJ/mol Temp (oC) 80 C 60 40 q = m(SH)(T) 20 B Heat of fusion 6.02 kJ/mol 0 A -20 E o SH ice = 2.1 J/g C o SH liq = 4.18 J/g C o SH gas = 2.0 J/g C -40 0 10 20 30 kilojoules of heat added 40 50 60 Question • Explain why orange growers spray their trees with water when there is a threat of freezing temperatures. Question • Why does steam at 100ºC cause more severe burns than water at the same temperature?