Gases - Saint Joseph High School
advertisement
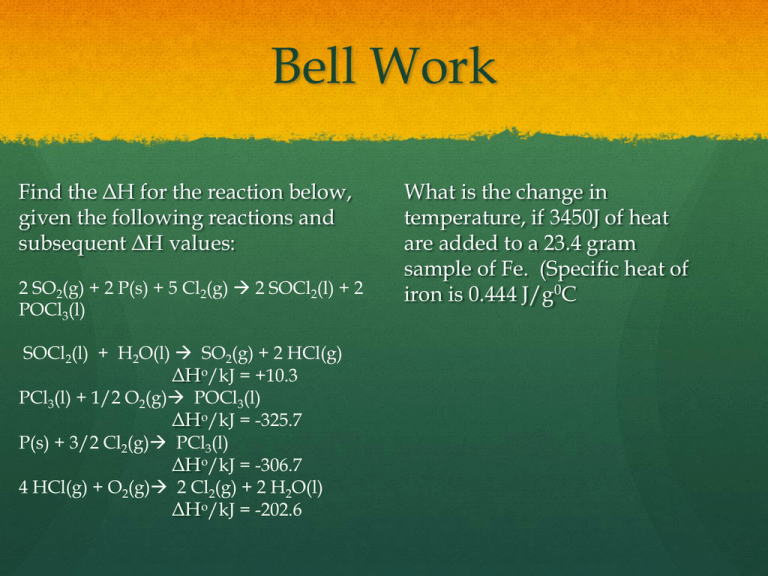
Bell Work Find the ΔH for the reaction below, given the following reactions and subsequent ΔH values: 2 SO2(g) + 2 P(s) + 5 Cl2(g) 2 SOCl2(l) + 2 POCl3(l) SOCl2(l) + H2O(l) SO2(g) + 2 HCl(g) ΔHo/kJ = +10.3 PCl3(l) + 1/2 O2(g) POCl3(l) ΔHo/kJ = -325.7 P(s) + 3/2 Cl2(g) PCl3(l) ΔHo/kJ = -306.7 4 HCl(g) + O2(g) 2 Cl2(g) + 2 H2O(l) ΔHo/kJ = -202.6 What is the change in temperature, if 3450J of heat are added to a 23.4 gram sample of Fe. (Specific heat of iron is 0.444 J/g0C Gases Chapter 13 Properties Uniformly fills a container Easy to compress Mixes completely with other gases Exerts pressure on surroundings Atmospheric pressure results from the mass of the air being pulled toward the center of the earth by gravity---weight of the air. Units of pressure mmHg (millimeters of mercury) Torr Torr and mmHg can be used interchangeably Atm (standard atmosphere) Pascal Kinetic-Molecular theory Gases can be compressed easily, but liquids and solids cannot be compressed because particles are already close together The intermolecular forces between gases are weak. Gas particles move until a collision alters their course Energy added into the system increases the kinetic energy of the particles, which translates to an increase in temperature of the gas. Relationship between pressure and volume Boyle’s law- pressure times volume equals a constant P1V1=P2V2 Example of Boyle’s Law A container holds 500. mL of CO2 at 20.° C and 742 torr. What will be the volume of the CO2 if the pressure is increased to 795 torr? V1=500mL P1=742 torr V2= x P2=795 torr (500mL)(742Torr)=(x)(795Torr) X=470mL Practice Problem A gas tank holds 2785 L of propane, C3H8, at 830. mm Hg. What is the volume of the propane at standard pressure, 760mmHg? V1P1 = V2P2 (2785L)(830mmHg)=(x)(760mmHg) 3042L Relationship between volume and temperature Charles’s Law-gas volume is directly proportional to the temperature V1 = V2 T1 T 2 Temperature is in Kelvin (+273) Example of Charles’s Law A container holds 50.0 mL of nitrogen at 25° C and a pressure of 736 mm Hg. What will its volume be, if the temperature increases by 35° C? V1 = V2 T1 T2 50mL = x 298K 333K x= 56mL Practice problem A sample of helium has a volume of 521 dm3 at a pressure of 75 cm Hg and a temperature of 18° C. When the temperature is increased to 23° C, what is the volume of the helium? V1 = V2 T1 T2 521dm3 = x 291K 296K =530dm3 Combined gas Law P1V1 = P2V2 T1 T2 The combined gas law is a mix between Charles’s, Boyle’s, and Gay-Lussac’s Laws Example A sample of argon has a volume of 5.0 dm3 and the pressure is 0.92 atm. If the final temperature is 30.° C, the final volume is 5.7 L, and the final pressure is 800. mm Hg, what was the initial temperature of the argon? P1V1 = P2V2 T1 T2 (5.0dm3)(0.92atm) = (5.7L)(1.05atm) x 303K =233K Practice problem A sample of sulfur dioxide occupies a volume of 652 mL at 40.° C and 720 mm Hg. What volume will the sulfur dioxide occupy at STP? P1V1 = P2V2 T1 T2 (652mL)(720mmHg) = (x)(760mmHg) 313K 273K 539mL Avogadro’s Law States that the volume of gas is proportional to the number of gas particles at constant temperature and pressure. I.E. A volume occupied by one mole of gas is 22.4L when the temperature of the gas is at 0oC and its pressure is at 1atm. These conditions are referred to as standard temperature and pressure (STP) STP conditions standards listed below 22.4 Liters 1 atm=760mmHg or Torr 273 K Example of STP Calculate the number of moles of nitrogen gas produced and grams of sodium azide consumed if 115L of N2 results from a sodium azide explosion. 115L x 1mol 22.4L =5.13 moles Ideal gas Law PV=nRT P-pressure(atm) V-volume(L) n-number of moles (mol) R-constant (0.0821L*atm/mol *K) T-temperature (Kelvin) Example A reaction yields 3.75 L of nitrogen monoxide. The volume is measured at 19°C and at a pressure of 1.10 atm. What mass of NO was produced by the reaction? PV=nRT (1.10atm)(3.75L) =x(0.0821L*atm/mol*K)(292K) 0.17moles 0.17mol x 30g/mol 5.1 g Practice problem What is the pressure inside a tank that has a volume of 1.20 x 103 L and contains 12.0 kg of HCl gas at a temperature of 18°C? PV=nRT (x)(1.20x103L)=(329mol)(0.0821L*atm/mol*K)(291K) 6.6atm











