Molar Mass.ppt - christophersonbiology
advertisement

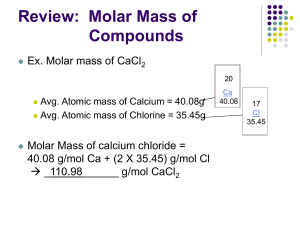
Wake-up 1. Study Polyatomic Ions for about 5/10 minutes. Write NO WAKE-UP on Friday MOLAR MASS What is molar mass? How do you calculate molar mass? Mole The unit for a specific amount of a substance/particle Avogadro’s Number 1 mole = 6.02 x 1023 particles Types of particles measured by Moles Atoms, elements, covalent compounds or ionic compounds Mole Road Map Practice Problem 1. How many cadmium atoms are there in 6.57x103 moles? Practice Problem 2. How many moles of nitrogen are there in 4.3x1023 molecules? Mole Road Map Moles to Mass OR Molar Mass Molar Mass The sum of the atomic weights of all the atoms in a molecule Molar Mass Units Grams Molar Mass (MM) = (g/mol) Moles Use Periodic Table to determine MM If there was one mole of Sodium (Na), it’s molar mass would be ________________ g/mol. • Carbon • Mercury • Iron MM= 12.0 g / mol MM= 200.6 g / mol MM=55.8 g / mol How many particles do these elements contain? Particles: 1. Atoms 2. Formula Units 3. Molecules How do you calculate molar mass of compounds? • • • • • • SO 3 EX. Sulfur Trioxide Chemical Formula = ___________ How many total atoms make up one molecule? 1+3=4 Total Atoms = _____________ 1 x ______ 32.1 = 32.1 S: ___ 16.0 3 x ______ O: ___ = 48.0 48.0 32.1 Molar Mass: __________ + ____________ 80.1 • The molar mass of sulfur trioxide = _______ g/mol Your Turn PCl3 • EX. Phosphorus Trichloride Chemical Formula = _________ 1+3=4 • How many total atoms make up one molecule? Atoms = _______ 1 x ______ 31.0 31.0 = • P: ___ 3 x ______ 35.5 = _________ 106.5 + • Cl: ___ 137.5 grams per 1 mol • Sodium Hydrogen Carbonate NaHCO3 MM = 84.0 g / mol Conversions using Molar Mass 1. How many grams are in 4.5 moles of sodium fluoride? Conversions using Molar Mass 2. How many moles are in 3.4 x 10-7 grams of silicon dioxide? Conversions using Molar Mass 3. How many moles are in 68 grams of Copper (II) hydroxide? Conversions using Molar Mass 4. How many grams are in 2.3 x 10-4 moles of Calcium phosphate?