Document
advertisement

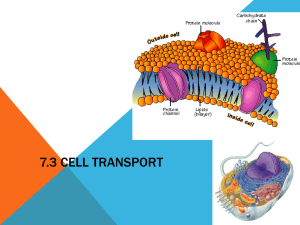
Membrane Transport Cell membranes • The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. • They are not passive barriers. They control the structures and environments of the compartments they define, and thereby the metabolism of these compartments. The membrane itself is a metabolic compartment with unique functions. Membrane functions • surrounds the cytoplasm of a cell and physically separates the intracellular components from the extracellular environment • anchors the cytoskeleton (a cellular 'skeleton' made of protein and contained in the cytoplasm) and gives shape to the cell • attachs the cell to the extracellular matrix and other cells to help group cells together to form tissues • provides mechanisms for cell-to-cell communication • is differentially permeable and regulates what enters and exits the cell, thus facilitating the transport of materials needed for survival • maintains the cell potential Structural organisation of cell membrane • The membrane consists of a lipid bi-layer. Phopholipids, the main lipid component, are oriented in such way that the hydrophobic non-polar tails are pointing towards each others while the hydrophylic polar phosphate heads point toward the cytosolic (internal) and external surface. This creates an hydrophobic barrier around the cell. Phopholipid • Phospholipids are composed of two aliphatic chains and contain a phosphate group on the head Fluid mosaic model • Other components of the membrane are other lipids (e.g. cholesterol, glycolipids) and proteins. Body Solutions • Intra cellular fluid (ICF)‐ within cells • Extra cellular Fluid (ECF)‐ outside cells Inter cellular = tissue fluid = interstitial fluid Plasma = fluid portion of blood • Composition of fluids change as substances move between compartments nutrients, oxygen, ions and wastes move in both directions across capillary walls and cell membrane Relative permeability of a phospholipid bilayer to various substances Type of substance Examples Behaviour Gases CO2, N2, O2 Permeable Small uncharged polar molecules Urea, water, ethanol Permeable, totally or partially Large uncharged polar molecules glucose, fructose Not permeable Ions K+, Na+, Cl-, HCO3- Not permeable Charged polar molecules ATP, amino acids, glucose-6-phosphate Not permeable Selective Permeability of Membrane • Lipid bilayer permeable to nonpolar, uncharged molecules oxygen, CO2, steroids permeable to water which flows through gaps that form in hydrophobic core of membrane as phospholipids move about • Transmembrane proteins act as specific channels: small and medium polar & charged particles • Macromolecules unable to pass through themembrane vesicular transport Gradients Across the Plasma Membrane • Membrane can maintain difference in concentration of a substance inside versus outside of the membrane (concentration gradient) more O2 & Na+ outside of cell membrane more CO2 and K+ inside of cell membrane • Membrane can maintain a difference in charged ions between inside & outside of membrane (electrical gradient or membrane potential) • Substances always move down their concentration gradient and towards the oppositely charged area ions have electrochemical gradients Membrane Transport • Passive transport mechanisms requires no ATP random molecular motion of particles provides the necessary energy filtration, diffusion, osmosis • Active transport mechanisms consumes ATP active transport and vesicular transport Thermodynamics • A physiological process can only take place if it complies with basic thermodynamic principles. A general principle of thermodynamics that governs the transfer of substances through membranes is that the exchange of free energy, ΔG, for the transport of a mole of a substance of concentration C1 in a compartment to another compartment where it is C2 present at C2 G RT ln C1 • C2 is less than C1 ΔG is negative, and the process is thermodynamically favorable. Chemical potential Electrochemical potential U k k G k k S ,V , l k const T , P, l k const 0 RT ln C ~ 0 RT ln C ZF Passive transport • Teorell equation describes the motion of chemical species in a fluid medium d~ j UC dx j where S t j is the "diffusion flux" (amount of substance per unit area per unit time), U is mobility of particles, C is the concentration • Nernst–Planck equation describes the flux of ions under the influence of both an ionic concentration gradient and an electric field dC d j URT UCZF dx dx where Simple Diffusion • Simple Diffusion – the net movement of particles from area of high concentration to area of low concentration (Down gradient) due to their constant, spontaneous motion. Fick's law dC j URT dx where D is the diffusion coefficient D URT dC j D dx Diffusion Rates Factors affecting diffusion rate through a membrane • temperature ‐ ↑ temp., ↑ motion of particles • molecular weight ‐ larger molecules move slower • steepness of concentrated gradient ‐ ↑ difference, ↑ rate • membrane surface area ‐ ↑ area, ↑ rate • membrane permeability ‐ ↑ permeability, ↑ rate Facilitated diffusion • It also called carrier-mediated diffusion, is the movement of molecules across the cell membrane via special transport proteins that are embedded within the cellular membrane. Many large molecules, such as glucose, are insoluble in lipids and too large to fit through the membrane pores. Therefore, it will bind with its specific carrier proteins, and the complex will then be bonded to a receptor site and moved through the cellular membrane. Facilitated diffusion • transport of solute through a membrane down its concentration gradient • Does not consume ATP • Solute attaches to binding site on carrier, carrier changes confirmation, then releases solute on other side of membrane • Saturation occurs in facilitated diffusion because not enough carriers may be available to handle all the free solute molecules. The rate of movement may reach a maximum. Membrane Carriers • Uniport carries only one solute at a time • Symport carries 2 or more solutes simultaneously in same direction (cotransport) • Antiport carries 2 or more solutes in opposite directions (countertransport) sodium‐potassium pump brings in K+ and removes Na+ from cell • Carriers employ two methods of transport facilitated diffusion active transport Osmosis • If two solutions of different concentration are separated by a semipermeable membrane which is permeable to to the smaller solvent molecules but not to the larger solute molecules, then the solvent will tend to diffuse across the membrane from the less concentrated to the more concentrated solution. This process is called osmosis. Osmosis • Osmosis ‐ flow of water from one side of a selectively permeable membrane to the other from side with higher water concentration to the side with lower water concentration • reversible attraction of water to solute • particles forms hydration spheres • makes those water molecules less • available to diffuse back to the side • from which they came • Aquaporins ‐ channel proteins • specialized for passage of water Filtration • is movement of water and solute molecules across the cell membrane due to hydrostatic pressure generated by the cardiovascular system. Depending on the size of the membrane pores, only solutes of a certain size may pass through it. For example, the membrane pores of the Bowman's capsule in the kidneys are very small, and only albumins, the smallest of the proteins, have any chance of being filtered through. On the other hand, the membrane pores of liver cells are extremely large, to allow a variety of solutes to pass through and be metabolized. Active Transport • Active transport – carrier‐mediated transport of solute through a membrane up (against) its concentration gradient • ATP energy consumed to change carrier • Examples of uses: • sodium‐potassium pump keeps K+ concentration higher inside the cell bring amino acids into cell pump Ca2+ out of cell Primary and secondary active transport • Active transport is the movement of a substance against its concentration gradient (from low to high concentration). In all cells, this is usually concerned with accumulating high concentrations of molecules that the cell needs, such as ions, glucose and amino acids. If the process uses chemical energy, such as from adenosine triphosphate (ATP), it is termed primary active transport. Secondary active transport involves the use of an electrochemical gradient. Primary active transport • It also called direct active transport, directly uses energy to transport molecules across a membrane. • Most of the enzymes that perform this type of transport are transmembrane ATPases. A primary ATPase universal to all life is the sodium-potassium pump, which helps to maintain the cell potential. Secondary active transport • In co-transport, energy is used to transport molecules across a membrane; however, in contrast to primary active transport, there is no direct coupling of ATP; instead, the electrochemical potential difference created by pumping ions out of the cell is used. • The two main forms of this are antiport and symport. Mechanism • The pump, with good binds ATP, binds 3 intracellular Na+ ions. • ATP is hydrolyzed, leading to phosphorylation of the pump at a highly conserved aspartate residue and subsequent release of ADP. • A conformational change in the pump exposes the Na+ ions to the outside. The phosphorylated form of the pump has a low affinity for Na+ ions, so they are released. • The pump binds 2 extracellular K+ ions. This causes the dephosphorylation of the pump, reverting it to its previous conformational state, transporting the K+ ions into the cell. • The unphosphorylated form of the pump has a higher affinity for Na+ ions than K+ ions, so the two bound K+ ions are released. ATP binds, and the process starts again. Sodium‐Potassium Pump • Each pump cycle consumes one ATP and exchanges three Na+ for two K+ • Keeps the K+ concentration higher and the Na+ concentration lower with in the cell than in ECF • Necessary because Na+ and K+ constantly leak through membrane half of daily calories utilized for Na+ ‐ K+ pump Ion concentration gradient Functions of Na+ ‐K+ Pump • Regulation of cell volume “fixed anions” attract cations causing osmosis cell swelling stimulates the Na+‐ K+ pump to ↓ ion concentration, ↓ osmolarity and cell swelling • Secondary active transport steep concentration gradient maintained between one side of the membrane and the other – (water behind a dam) Sodium‐glucose transport protein (SGLT) – simultaneously binds Na+ and glucose and carries both into the cell does not consume ATP • Heat production thyroid hormone increase # of Na+ ‐ K+ pumps consume ATP and produce heat as a by‐product • Maintenance of a membrane potential in all cells pump keeps inside more negative, outside more positive necessary for nerve and muscle function Vesicular Transport • Vesicular Transport – processes that move large particles, fluid droplets, or numerous molecules at once through the membrane in vesicles – bubblelike enclosures of membrane motor proteins consumes ATP • Endocytosis –vesicular processes that bring material into the cell phagocytosis – “cell eating” ‐ engulfing large particles pseudopods phagosomes macrophages pinocytosis – “cell drinking” taking in droplets of ECF containing molecules useful in the cell pinocytic vesicle receptor‐mediated endocytosis – particles bind to specific receptors on plasma membrane clathrin‐coated vesicle • Exocytosis – discharging material from the cell • Utilizes motor proteins energized by ATP