Anticoagulants, Thrombolytics Agents and Antiplatelet Drugs
advertisement

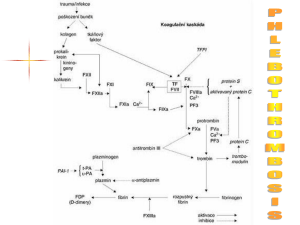
Antithrombin III Independent Anticoagulants Benedict R. Lucchesi, M.D., Ph.D. Department of Pharmacology University of Michigan Medical School Antithrombin III Independent Anticoagulants • Hirudin – From the medicinal leech – Synthesized by recombinant DNA techniques – Direct inhibitor of thrombin • Lepirudin (Refludin™) – desulfohirudin - a recombinant hirudin* derived from yeast cells. • Hirugen (bivalirudin, Angiomax™) – Synthetic dodecapeptide derived from hirudin. • Argatroban – Arginine based compound – Weak competitive inhibitor of thrombin. • These compounds are used in those patients who have developed thrombocytopenia during treatment with heparin. Lepirudin (Refludin™) Lepirudin [rDNA] (Refludan™) • • • • • • highly specific direct inhibitor of thrombin - [Leu1-Thr2]-63desulfohirudin - a recombinant hirudin* derived from yeast cells. polypeptide - 65 amino acids MW=6979.5 identical to natural hirudin except for the substitution of leucine for isoleucine at the N-terminal end of the molecule and the absence of a sulfate group on tyrosine 63. action independent of ATIII and not inhibited by platelet factor 4 one molecule of lepirudin binds one molecule of thrombin - all thrombin-dependent coagulation pathways are affected approved for clinical use in the treatment of heparin-induced thrombocytopenia type II. * Hirudin derived from the leech Hirudo medicinalis Bivalirudin (Hirulog, ™ Angiomax ) Bivalirudin (Hirulog, Angiomax™) • Synthetic 20-amino acid peptide analog of naturally occurring hirudin • Bivalirudin is a specific and reversible direct thrombin inhibitor that binds to the catalytic site and the anionbinding exosite of circulating and clot-bound thrombin. • Inhibition of thrombin prevents activation of factors V, VIII, and XIII; conversion of fibrinogen to fibrin; platelet activation and aggregation). Bivalirudin (Hirulog, Angiomax™) • The effects of bivalirudin are reversed as thrombin slowly • • cleaves the bilvalirudin-Arg3-Pro4 bond, resulting in recovery of thrombin active site function. The onset of anticoagulant effect is immediate after direct IV injection of bivalirudin. Bivalirudin therapy prolongs several coagulation assays: – activated clotting time (ACT), – activated partial thromboplastin time (aPTT), – thrombin time (TT), – prothrombin time (PT) – Coagulation times return to the normal range approximately 1—2 hours after discontinuance of the drug. • (A) Structure of bivalirudin and (B) bivalirudin / hirudin complexes • Bivalirudin consists of an active site-directed moiety linked by a poly-glycine spacer to a dodecapeptide analogue of the carboxy terminal of hirudin. • Once bivalirudin complexes thrombin, it is converted from a noncompetitive inhibitor that interacts with both the active site and exosite 1 on thrombin to a competitive inhibitor that only binds to exosite 1. • Potential for enhanced protein C activation by bivalirudin. Fluidphase thrombin is complexed and inhibited by bivalirudin. • Upon arrival to the microcirculation where thrombomodulin is concentrated, the amino-terminal domain of bivalirudin is released, leaving only the carboxy-terminal domain bound to exosite 1 on thrombin. • The affinity of thrombomodulin for thrombin is higher than that of the carboxy-terminal dodecapeptide of bivalirudin, thus thrombin binds to thrombomodulin, where it activates protein C. Argatroban Argatroban • Argatroban, a synthetic piperidine carboxylic acid derivative of l-arginine, is an anticoagulant. • Commercially available argatroban is a racemic mixture of the R- and S-diastereoisomers in a ratio of approximately 65 to 35, with the S-isomer having about twice the thrombin-inhibitory potency of the R-isomer. • Argatroban, a highly selective, reversible, small-molecule direct thrombin inhibitor that binds rapidly to the catalytic site/a polar region of both circulating (free) and clot-bound thrombin. Argatroban • Inhibition of thrombin prevents various steps in the • • • coagulation process (e.g., activation of factors V, VIII, and XIII and of protein C; conversion of fibrinogen to fibrin; platelet activation and aggregation). At infusion rates up to 40 mcg/kg per minute, argatroban produces dose-dependent increases inactivated partial thromboplastin time (aPTT) and several other coagulation assays (activated clotting time [ACT], prothrombin time [PT], and thrombin time [TT]). Metabolized principally by the liver via hydroxylation and aromatization. Does not induce antibody formation to itself nor does it interact with heparin-induced antibodies. Argatroban • Administered by continuous IV infusion. • Before administering argatroban, all parenteral anticoagulants must be discontinued and a baseline activated partial thromboplastin time (aPTT) obtained. Supplied, as a concentrated drug (100 mg/ml), which must be diluted 100-fold prior to infusion. Should not be mixed with other drugs prior to dilution in a suitable intravenous fluid.