Metabolism
advertisement

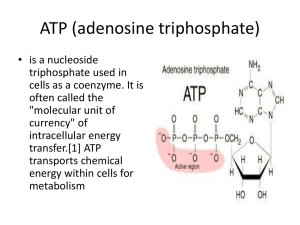
Microbial Metabolism Microbiology 2314 Why Study Bacterial Metabolism? • • • • • • For Interest Treatment of Disease Productive Use of Bacteria Unity of Biochemistry Inexpensive Easy to Grow and Study Energy • • • • Capacity to Do Work Calories / Joules Defined in Terms of What It Does 1 calorie = 4.184 Joules Cells Require Energy • Phototrophs Light • Chemolithotrophs Inorganic Chemicals • Chemoorganotrophs ? Types of Energy • Kinetic - Energy of Motion - Light, Heat, Mechanical • Potential - Stored Energy - Battery, Molecular Bonds, Reservoir Water Behind Dam Metabolism Metabolism is the sum total of all the chemical reactions within a living organism. Metabolism = Anabolism + Catabolism Anabolism • • • • • Make New Molecules Simple to Complex Assimilative Biosynthetic Endergonic/Endothermic Catabolism • • • • • Used to Obtain Energy Complex to Simple Degradative Dissimulative Exergonic/Exothermic The energy of catabolic reactions is used to drive anabolic reactions. Energy is typically stored as ATP. ATP The universal energy carrying molecule in living organisms. • 1 Adenine • 3 Phosphates • Ribose Energy Flows One-Way Through a System The Earth’s Energy Comes From the Sun Rules of Metabolism • Concerned with Acquiring and Using Energy. • Efficient Energy Users Survive and Reproduce Their Genes. Their Advantage is Passed On. • Not Magic. Follows Simple Physical Laws. • To Understand Metabolism – Must Understand the Components of Metabolism. Regulation • Living Organisms are Very Complicated. • Life Requires a High Level of Regulation • Regulation is Achieved Via Molecular Recognition • Recognition Preprogrammed Response The Principle of Molecular Recognition • • • • • • Specialized Cells Receptors are Binding Sites Ligands are Docking or Attaching Sites Binding by Ligands to Receptors Message Transfer to the Cell’s Interior Control Center Initiates a Response Information About Enzymes • Enzymes – Proteins Produced by Living Cells – Catalyze Chemical Reactions – Lower the Activation Energy • Enzymes as Proteins – – – – Globular Proteins Efficient Operate at Low Temperatures Subject to Cellular Control Information About Enzymes • Enzymes are the Tools of Life – Performs Functions Necessary for Life – Has Only One Job – Do Everything in the Cell • Chemical Nature of Enzymes – Determined by the Chemical Characteristics of the Amino Acids that Compose it – Influenced by the Arrangement of the Amino Acids Amino Acids • 20 Amino Acids • Arranged in Chain During the Process of Translation • Strong Covalent Peptide Bonds • Proteases Break Bonds Types of Amino Acids 1. Hydrophobic • • Repelled by Water Only Associate with One Another 2. Basic • • Bind Protons / Positively Charged Attracted by Water 3. Acidic • • Negatively Charged Attracted by Water 4. Polar-Nonionizable • • No Charge Attracted by Water Naming Enzymes • End in ASE • Named According to Substrate or Type of Reaction they Catalyze • Six Classes of Enzymes Enzyme Components CoFactor Compostion • Metal Ion - Fe, Cu, Mg, Mn, Ca, Zn • Coenzyme - NAD, NADP, FAD, Coenzyme A • Vitamins are Organic Cofactors • Minerals are Inorganic Cofactors Enzyme Characteristics • • • • • • Organic Catalysts Renewable Large Work Quickly Stable and Long Lasting Unique Functional Structure Enzymes serve as Catalysts. Catalysts lower the activation energy making it easier for reactions to occur. The Mechanism of Enzymatic Action • An enzyme attracts substrates to its active site, catalyzes the chemical reaction by which products are formed, and then allows the products to dissociate—i.e., separate from the enzyme surface. • The combination formed by an enzyme and its substrates is called the enzyme–substrate complex. • When two substrates and one enzyme are involved, the complex is called a ternary complex; one substrate and one enzyme are called a binary complex. • The substrates are attracted to the active site by electrostatic and hydrophobic forces Lock and Key Paradigm 1. Interaction Between Enzyme and Substrate 2. Initiates a Preprogrammed Reaction 3. Causes Substrate Transformation Denaturation Factors Influencing Enzymatic Activity 1. Temperature 2. pH 3. Concentration Inhibition • Competitive Inhibitors - Compete with the normal substrate for active site of the enzyme - Mimic the true substrate • Noncompetitive Inhibitors - Act on other parts of the apoenzyme or on the cofactor and decrease the enzymes ability to combine with the normal substrate Inhibition Feedback Inhibition / Estrogen Production • • • • • • Hypothalamus Secretes Gonadotrophin Gonadotrophin goes to Pituitary Causes Pituitary to release LH and FSH LH and FSH act on the Overies Overies release Estrogen Estrogen goes to the Hypothalamus Knowing What We Do About Enzymes, Explain Why a Siamese Cat is Colored the Way They Are. Glycolysis • Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. • The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide). Glycolysis is a metabolic pathway found in the cytosol of cells in all living organisms both aerobic and anaerobic. • When oxygen is present, acetyl-CoA is produced from the pyruvate molecules created from glycolysis. • Once acetyl-CoA is formed, two processes can occur, aerobic or anaerobic respiration. • When oxygen is present, the mitochondria will undergo aerobic respiration which leads to the Krebs cycle. • However, if oxygen is not present, fermentation of the pyruvate molecule will occur. Kreb’s Cycle • In the presence of oxygen, when acetyl-CoA is produced, the molecule then enters the Krebs cycle inside the mitochondrial matrix, and gets oxidized to CO2 while at the same time reducing NAD to NADH. NADH can be used by the electron transport chain to create further ATP as part of oxidative phosphorylation. Summary Step coenzyme yield Glycolysis preparatory phase ATP yield -2 4 Glycolysis pay-off phase Oxidative decarboxylation of pyruvate 2 NADH 4 2 NADH 6 2 Krebs cycle 6 NADH 2 FADH2 Total yield 18 4 36 ATP Source of ATP Phosphorylation of glucose and fructose 6-phosphate uses two ATP from the cytoplasm. Substrate-level phosphorylation Oxidative phosphorylation Each NADH produces net 2 ATP due to NADH transport over the mitrochondrial membrane Oxidative phosphorylation Substrate-level phosphorylation Oxidative phosphorylation Oxidative phosphorylation From the complete oxidation of one glucose molecule to carbon dioxide and oxidation of all the reduced coenzymes. No Oxygen? • Without oxygen, pyruvate (pyruvic acid) is not metabolized by cellular respiration but undergoes a process of fermentation. • The pyruvate is not transported into the mitochondrion, but remains in the cytoplasm, where it is converted to waste products that may be removed from the cell. • This serves the purpose of oxidizing the electron carriers so that they can perform glycolysis again and removing the excess pyruvate. • This waste product varies depending on the organism. In skeletal muscles, the waste product is lactic acid. This type of fermentation is called lactic acid fermentation. • In yeast, the waste products are ethanol and carbon dioxide. • This type of fermentation is known as alcoholic or ethanol fermentation. • The ATP generated in this process is made by substrate-level phosphorylation, which does not require oxygen. Efficiency? • Fermentation is less efficient at using the energy from glucose since 2 ATP are produced per glucose, compared to the 38 ATP per glucose produced by aerobic respiration. • This is because the waste products of fermentation still contain plenty of energy. Ethanol, for example, can be used in gasoline solutions. Chemiosmotic Model of ATP Generation • Chemiosmosis is the movement of ions across a selectively-permeable membrane, down their electrochemical gradient. • More specifically, it relates to the generation of ATP by the movement of hydrogen ions across a membrane during cellular respiration. • Hydrogen ions (protons) will diffuse from an area of high proton concentration to an area of lower proton concentration. • Peter Mitchell proposed that an electrochemical concentration gradient of protons across a membrane could be harnessed to make ATP. • He linked this process to osmosis, the diffusion of water across a membrane, which is why it is called chemiosmosis. • ATP synthase is the enzyme that makes ATP by chemiosmosis. • It allows protons to pass through the membrane using the kinetic energy to phosphorylate ADP making ATP. • The generation of ATP by chemiosmosis occurs in chloroplasts and mitochondria as well as in some bacteria. Compare/Contrast Aerobic and Anaerobic Respiration • Aerobic respiration requires oxygen. • Anaerobic respiration does not require oxygen • Aerobic respiration tends to create more ATP per glucose molecule and is thus more efficient than anaerobic. • Cellular respiration is an aerobic process that has 3 stages. 1. glycolysis is the anaerobic stage and does not require oxygen. 2. the krebs cycle or the citric acid cycle and chemiosmosis requires the use of oxygen. When there is no oxygen present, the cell is able to use only glycolysis and the process in which the cell recycles the NAD+ required in glycolysis to repeat glycolysis is called fermentation, an anaerobic process. • there are 2 types: 1. alcoholic fermentation 2. lactic fermentation • Alcoholic fermentation makes the byproduct: alcohol. • Yeast and other prokaryotic organisms tend to use this type of fermentation. • Lactic fermentation makes the byproduct: lactic acid, hence the reason when you run, your legs have a burning sensation because your muscles are using the process of lactic fermentation and acid burns. **the lactic acid is ultimately brought to the liver and detoxified Summary • Aerobic = oxygen = more efficient (more ATP) • Anaerobic = no oxygen = less efficient (less ATP) • Aerobic = cellular respiration • Anaerobic = fermentation • Fermentation originally referred to the foaming that occured during the manufacture of wine and beer, a process at least 10,000 years old. • That the frothing results from the evolution of carbon dioxide gas was not recognized until the 17th century. Fermentation • Louis Pasteur in the 19th century used the term fermentation in a narrow sense to describe the changes brought about by yeasts and other microorganisms growing in the absence of air (anaerobically); he also recognized that ethyl alcohol and carbon dioxide are not the only products of fermentation. Ethanol Fermentation • Ethanol fermentation, also referred to as alcoholic fermentation, is a biological process in which sugars such as glucose, fructose, and sucrose are converted into cellular energy and thereby produce ethanol and carbon dioxide as metabolic waste products. • Because yeasts perform this conversion in the absence of oxygen, ethanol fermentation is classified as anaerobic. • The chemical equation below summarizes the fermentation of glucose, whose chemical formula is C6H12O6. • One mole of glucose is converted into two moles of ethanol and two moles of carbon dioxide: C12H22O11 +H2O + invertase → C6H12O6 C6H12O6 + Zymase → 2C2H5OH + 2CO2 • C2H5OH is the chemical formula for ethanol. Lactic Acid Fermentation • Lactic acid fermentation is a biological process by which sugars such as glucose, fructose, and sucrose, are converted into cellular energy and the metabolic byproduct lactate. • It is an anaerobic fermentation reaction that occurs in some bacteria and animal cells, such as muscle cells, in the absence of oxygen. • The process of lactic acid fermentation using glucose is summarized below. In fermentation, one molecule of glucose is converted to two molecules of lactic acid: C6H12O6 → 2 CH3CHOHCOOH • Before lactic acid fermentation can occur, the molecule of glucose must be split into two molecules of pyruvate. This process is called glycolysis. Identifying Bacteria • Fermenting bacteria have characteristic sugar fermentation patterns, i.e., they can metabolize some sugars but not others. For example, Neisseria meningitidis ferments glucose and maltose, but not sucrose and lactose, while Neisseria gonorrhoea ferments glucose, but not maltose, sucrose or lactose. • Such fermentation patterns can be used to identify and classify bacteria