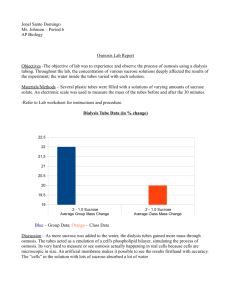
AP Lab 1 Osmosis and Diffusion
advertisement
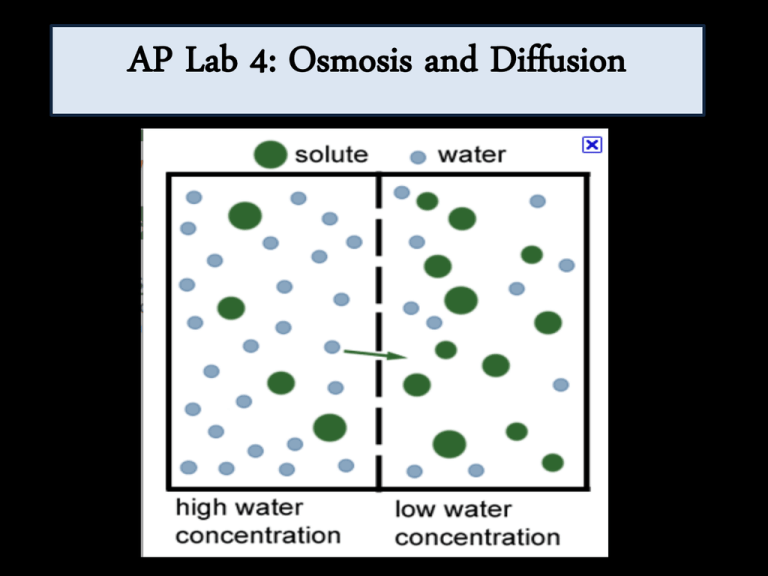
AP Lab 4: Osmosis and Diffusion Purpose 1. Investigate processes of diffusion and osmosis in a model membrane system, and 2. Investigate effect of solute concentration on water potential as it relates to living plant tissues. Key Concepts • Diffusion: The mvmt of materials from high to low concentrations…passive transport = no energy. • Osmosis: Mvmt of water from high to low. Key Concepts Key Concepts: Water Potential • Tendency of water to leave one place in favor of another. • PP: amt of pressure in cell • SP: solute concentration: As solute is added, value for solute potential becomes more negative decreases wp. Key Concepts: Molarity • 1 M Sucrose: 342.3 g/mol + 1000 mL (1 L) • 1 M NaCl: 58.44 g/mol + 1000 mL (1 L) • To make a 0.5 M, cut the grams amount by ½. Procedure Pt 1 • 1) Tie off one end of a wet dialysis bag. Fill with a 10 mL sugar/salt solution of a concentration (like 1M NaCl). Tie off and weigh. • Fill cup with diff. sugar/salt concentration (like 0.5M NaCl). • Let sit for 30 mins. • Reweigh Procedure Part 2 • 1) Obtain 6 wet dialysis bags. • 2) Fill with different concentrations of sucrose. Weigh to get initial weights (OM, 0.2 M, 0.4 M, 0.6 M, 0.8 M, 1 M) • 3) Place dialysis bags in distilled water. • 4) Wait and then weigh bags to see what happened. Graph results. • Hint: Be sure that the entire dialysis bag and tie are submerged! Procedure Part 3 • 1) Like Ex 2, but w/ potatoes. 1st weigh potatoes. • 2) Put potatoes (no skin) in solutions of varying sucrose concentrations. • 3) Calculate weight gain or loss. Procedure Part 4 • Use the value for the molar concentration of the potato cores that you obtain in Exercise 3 to determine the water potential for the potato cells. • Calculate water potential (pressure potential on open beaker is zero). Procedure Part 5 • Watch the effect of placing an onion cell into a solution that has a lower or higher concentration of water than the cell.