How Do Molecules Enter Cells Part II 2.1
advertisement

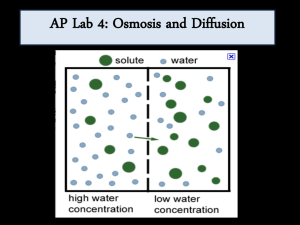
How Do Molecules Enter Cells…Part II Student learning targets: after this investigation you will be able to • Plan and conduct a scientific investigation, choosing a method appropriate to the question being asked. • Collect, analyze, and display data using calculators • Draw conclusions supported by evidence from the investigation and consistent with established scientific knowledge.*c • Give examples of common solutions. • Describe the structure of the cell membrane and how the membrane regulates the flow of materials into and out of the cell. • Provide practical examples of how this knowledge can be applied. • Survivorman (4:15, 4:25) Survival Brainstorm • Imagine that you are on a stranded life raft in the middle of the ocean like the previous video clip. • Write down at least two primary needs that need to be taken care for your survival. • Pair-share your ideas with a neighbor. Classshare. • Lets focus on Water…Write down how long do you think you could survive without water on the raft? Remember? • What were the molecules that we were dealing with in our Dialysis Tube Lab? • IKI, starch, glucose, water • Which one of the molecules did we not have evidence of it moving across the Dialysis Tube Membrane? • Water!! Water • Write at least two reasons why you think water is important for your body. • Pair-share then Class-share • If you could not find fresh water would it be a good idea to drink the salt water surrounding the life raft? Why or why not? Salt Water…What is it? • Suppose you are making salt water…write what is dissolving the salt? • This is called a solvent. • Whenever you have a solid, like salt, that is being added to water it is called a solute. • When a solvent and a solute are combined you now have a solution…so see if you have a solution to this question… – salt water is a ________________? Let’s see how well you can concentrate… • You take a 200 mL water sample from the mouth of the Snoqualmie river where it meets the ocean, the ocean itself, and the headwater of the Snoqualmie…rank in order which body of water has the most salt, and WHY? • The amount of a solute dissolved in a solvent in a fixed volume is known as its concentration. The concentration of a solution can be measured in ppm (parts per billion), ppb, % concentration, Molarity or g/L. • Review your ranked order from the example above and now explain “why” in terms of the concept of concentration.. Back to the dialysis tube investigation • How could you test for whether or not water moved into or out of the dialysis tube membrane? _________________________ Investigation question: does solute concentration effect the movement of water into or out of cells? • Write your hypothesis: - remember this is a testable statement • Write your prediction: If, then, because statement. • What would be the – manipulated-include all treatments – responding and – controlled (at least 3) variables of this investigation be? Responding variable- what are you measuring? • Here is an example of change in mass…between Coleen and Jerry who would win this weeks weigh off in the Biggest Loser and WHY? • That’s right…Coleen would barely win with a weight loss of 4.6% versus 4.5% for Jerry…how do we calculate % loss? % Change (∆) • [(mass final - mass initial) / (mass initial) ] x 100 • What is Ed’s % Change for this episode of Biggest Loser? • What is Aubrey’s % change in weight? • Did you include the negative symbol in front of the value? [(mass final – mass initial) / (mass initial) ] x 100% • 203 lbs. - 200lbs. = 1.5% 200 lbs. Manipulated variable and levels of treatment. Controlled variables? Procedure • Lab Groups 1-6: Use 0.0M & 0.2M Sucrose Solution • Lab Groups 7-11: Use 0.4M & 0.6M Sucrose Solution • Lab Groups 12-16: Use 0.8M & 1.0M Sucrose Solution Procedure: 1. Gather 2 clear plastic cups & four pieces of potato core 2. Label Solute Concentration and group initials on the cups using the white 3. Completing the Virtual Cell Internet stickers at your tableWorksheet: ____/10 3. Fill cups with 50mL with the assigned solutions 4. Using the scales at your table mass (and record) two potato pieces and place in your first solution. Then repeat for your second solution. 5. Place cups in designated area to sit for a 24 hour period. Create the following Data Table: