Osmosis - Hicksville Public Schools / Homepage
advertisement

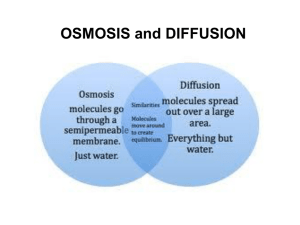
Aim: How do Osmosis and Diffusion compare? DN: Explain the difference between passive and active transport. HW: Page 199 #33-34 What is the diffusion of water called? Osmosis OSMOSIS The movement of water from an area of high concentration to an area of low concentration. What is meant by concentration? The amount of solute! Solvent: The liquid (ex. Water) Solute: The solid added to the liquid (ex. Salt/sugar) Distilled water 100% pure water Concentration Differences: 1) A 90% salt solution vs. a 20% salt solution Which solution has more salt particles? Which solution has more water molecules? 2) A 60% salt solution vs. 80% salt solution? Which container has a greater concentration of water molecules? = Solute (salt) = Solvent (water) Which container has a greater concentration of solute molecules? In which direction is osmosis going to occur? = Solute (sugar) = Solvent (water) Selectively Permeable Why does this happen? There is MORE WATER on the left side of the membrane so it moves to the right side. Why are the water molecules diffusing to the left? Water molecules are red The water is moving from an area of high to low! Why does water go in and out of the cell? EQUILIBRIUM If soluteTo is maintain equal, DYNAMIC then so is water amt., molecules still move back and forth! What happens to an animal cell if it is surrounded by a high concentration of water? Water will enter the cell! Cell will swell and burst! What happens to an animal cell if it is surrounded by a low concentration of water? The Cell will shrink!! Salt sucks!!!! Summary Questions Will osmosis happen? In which direction will osmosis happen? How is osmosis a form of passive transport? Osmosis is the movement of water from an area of high concentration to an area of low concentration. No ATP