Regents Warm Up
advertisement
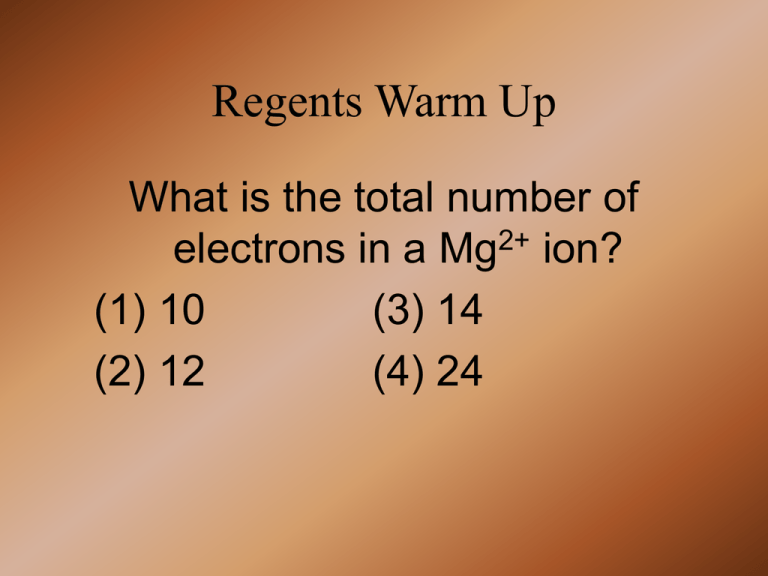
Regents Warm Up What is the total number of electrons in a Mg2+ ion? (1) 10 (3) 14 (2) 12 (4) 24 ELECTROCHEMISTRY AIM: What is an Electrolytic Cell? Do Now: What is a salt bridge? Voltaic Cell Review • What kind of Redox rxn occurs in all VOLTAIC Cells? • What occurs in the left half-cell? • What is the electrode in the right half-cell called? • Where are electrons generated? Which statement is true about oxidation and reduction in an electrochemical cell? 1. Both occur at the anode. 2. Oxidation occurs at the anode and reduction occurs at the cathode. 3. Both occur at the cathode. 4. Oxidation occurs at the cathode and reduction occurs at the anode Electrolytic Cell • Electrolytic Cells: Electrochemical Cells used to FORCE non-spontaneous redox reactions to occur. • They do NOT generate electrical energy – they USE it! • An electric current is applied to the cell to make it run – this is called ELECTROLYSIS • Electrolytic Cells are similar to Voltaic Cells • They both have: 1. Oxidation occuring at the Anode 2. Reduction occuring at the Cathode 3. Electrons travel from the Anode to the Cathode Electrolytic Cells • The difference is that in electrolytic cells the electrons are forced to move from the anode to the cathode A voltaic cell differs from an electrolytic cell in that in a voltaic cell 1. energy is produced when the reaction occurs 2. both oxidation and reduction occur 3. energy is required for the reaction to occur 4. neither oxidation nor reduction occurs Electrolytic Cell • Main Uses Include: 1. Producing Substances (Electrolysis) 2. Purifying Metals 3. Electroplating Which process occurs at the anode in an electrochemical cell? 1. the loss of protons 2. the gain of protons 3. the loss of electrons 4. the gain of electrons Practice Where does oxidation occur in an electrochemical cell? Which statement is true for any electrochemical cell? 1. at the cathode in both an electrolytic cell and a voltaic cell 3. at the anode in both an electrolytic cell and a voltaic cell 2. at the cathode in an electrolytic cell and at the anode in a voltaic cell 4. at the anode in an electrolytic cell and at the cathode in a voltaic cell 1. Oxidation occurs at the anode, only. 2. Oxidation occurs at both the anode and the cathode. 3. Reduction occurs at the anode, only. 4. Reduction occurs at both the anode and the cathode. Short Answer Question Aluminum is one of the most abundant metals in Earth's crust. The aluminum compound found in bauxite ore is Al2O3. Over one hundred years ago, it was difficult and expensive to isolate aluminum from bauxite ore. In 1886, a brother and sister team, Charles and Julia Hall, found that molten (melted) cryolite, Na3AlF6, would dissolve bauxite ore. Electrolysis of the resulting mixture caused the aluminum ions in the Al2O3 to be reduced to molten aluminum metal. This less expensive process is known as the Hall process. Explain, in terms of electrical energy, how the operation of a voltaic cell differs from the operation of an electrolytic cell used in the Hall process. Include both the voltaic cell and the electrolytic cell in your answer. ANSWER • A voltaic cell produces electrical energy by using a spontaneous redox reaction. An electrolytic cell uses electrical energy to drive a redox reaction that is not spontaneous. Short Answer Question 2 • Electroplating is an electrolytic process used to coat metal objects with a more expensive and less reactive metal. The accompanying diagram shows an electroplating cell that includes a battery connected to a silver bar and a metal spoon. The bar and spoon are submerged in AgNO3(aq). Explain the purpose of the battery in this cell. Answer Short Answer 2 • The electrolysis reaction is not a spontaneous reaction. Therefore, an external source of energy (the battery) is needed to drive the reaction. Batteries • There are 3 Types of Batteries 1. Dry Cell: do not contain a liquid, instead has a paste • Inner case is the anode • Carbon rod+paste work as cathode Batteries 1a) Primary Dry Cell: Uses a redox rxn that is NOT easily reversible Batteries 1b) Secondary Dry Cell: • Uses a redox reaction that is reversible • These are rechargable batteries Batteries 2) Lead Storage • Anode is Pb, Cathode is PbO2 • Typically uses H2SO4 • Operate well at extreme temps Batteries 3. Lithium Batteries • Li is easily oxidized and generates more voltage • Last longer then most other batteries











