Lecture I--introduction
advertisement
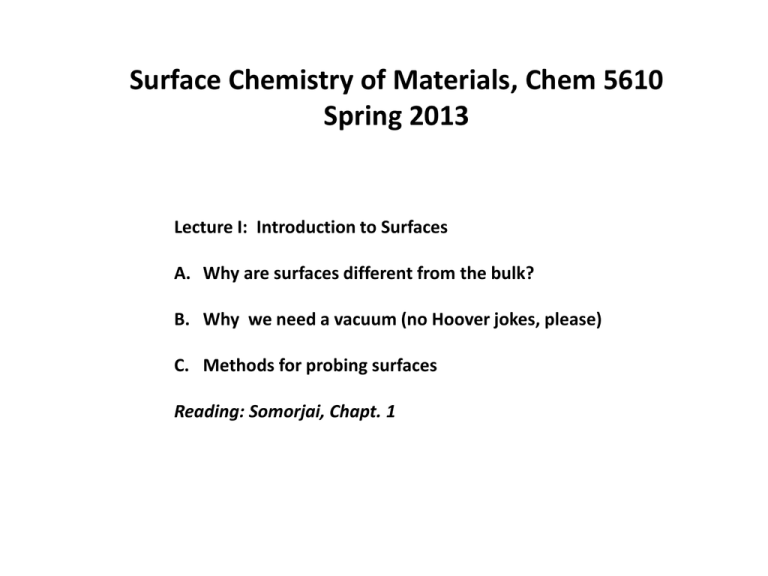
Surface Chemistry of Materials, Chem 5610 Spring 2013 Lecture I: Introduction to Surfaces A. Why are surfaces different from the bulk? B. Why we need a vacuum (no Hoover jokes, please) C. Methods for probing surfaces Reading: Somorjai, Chapt. 1 Why Surface Science? (1) Many important chemical reactions occur at outermost atomic layers of materials (typically, outermost 1-50 Å) Langmuir, H2 reactions at a W surface (1913) Haber, N2 + 3 H2 2 NH3 over iron catalyst (about the same time) The main drivers of surface science today: (A) Catalysis (B) Micro/nanoelectronics (C) Energy (photovoltaics, fuel cells…) And Tomorrow(?) Biological issues (tissue/prosethic compatibility, membrane chemistries…) Neuronetworks, biological and not 2 Atoms at a surface are low-coordinate relative to the bulk Surface atom, 5 bonds to nearest neighbors vacuum Surface Bulk Bulk atom, 6 bonds to nearest neighbors Unused surface bonds can interact, causing change in surface structure Surface dimerization Reconstruction of Si(100) A. Unreconstructed Si(100)-(1x1) surface. The Si atoms of the topmost layer are highlighted in orange; these atoms are bonded to only two other Si atoms, both of which are in the second layer (shaded grey). B. Reconstructed Si(100)-(2x1) surface. The Si atoms of the topmost layer form a covalent bond with an adjacent surface atom are thus drawn together as pairs; they are said to form "dimers". From 5 http://www.chem.qmul.ac.uk/surfaces/scc/scat1_6a.htm Surface is different electronically: Distribution of Surface Charge 1. Electron density trails off exponentially away from the surface into the vacuum 2. This partially depletes negative charge just below the surface Ion cores partially unshielded, net + charge Charge neutrality in the bulk Bulk Lang and Kohn, PRB 1 (1970) 4555 Region above surface negatively charged (several angstroms) Surface 6 Redistribution of Charge near surface sets up the Surface Dipole + + + - - + + Bulk 7 Work function is the extra energy needed to promote an electron from the HOMO (Fermi level) into the vacuum different for different surfaces e.g~ 4.3 eV, W ~ 5.3 eV, Pt EVacuum E Work Function EFermi 8 Why do we need a vacuum? O2 hydrocarbons H2O CO2 Atoms at the surface directly interact with gases in the environment Rxns occur at the surface that don’t occur in the bulk We need to control this Typical Atom Surface Density: ~ 1015 atoms/cm2 Flux of atoms of mass M to this surface from the gas phase (F) is given by (at gas temperature T) : F (atoms/cm2-sec) = 3.51 x 1022 P(Torr) x [M(g/mole) T]-1/2 (Somorjai) Note: At P = 3 x 10-5 Torr, M = 28 gr/mole; T = 300 K F ~ 1015 atoms/cm2-sec. Thus, assuming a “sticking coefficient” of 1, the surface is covered by a fresh monolayer every second under a mild vacuum Sticking Coefficient = probability/collision that an atom coming from the vacuum and colliding with the surface will stick! Sticking coefficients are often small (e.g., N2 on Au) but can approach 1 for , e.g., N2 on clean W. We need to keep surface contaminant concentrations low over the course of an experiment (~ 1 hour, say). Therefore, pressures ~ 10-9 or lower are required. This is known as ultra-high vacuum (UHV). Important: in measuring surface concentrations of adsorbed atoms, it is NOT pressure, but Pressure x Time [Exposure] that is important. 1 Langmuir = 10-6 Torr-sec is the standard unit of exposure Methods for maintaining and measuring ultrahigh vacuum (see standard texts, such as Briggs and Seah, Practical Surface Analysis: Chamber Materials: 304 Stainless steel now almost universal Pumps: (1) Turbomolecular pump with mechanical pump backing can go to ~ 10-10 Torr if careful. Typically ~ 10-9 Torr – 5 x 10-10 Torr advantage, can pump many different types of gases, rapid pump down from relatively high gas loadings back to UHV disadvantage, expensive, can malfunction during power outages, etc. (2) Ion pump with Ti Sublimator can maintain vacuums better than 5 x 10-11 Torr Fussy about what it will pump (O2, H2O good, CO bad) Relatively cheap, long lasting, restarts after power outages low pumping speeds, needs turbo to rough down from high gas loadings Other pumps include oil diffusion pumps, but not much used anymore. Measuring a vacuum: The “nude” (Bayard-Alpert) ion gauges A+ e- + A A+ + 2 ee- Filament emits electrons accelerated by grid Electrons ionize gas phase molecules Ions collected a grid. Grid current proportional to pressure. Ion gauges: Practical upper limit ~ 10-3 Torr Lower limit ~ 10-11 Torr Very reliable: They only fail during important experiments Methods for Probing Surfaces How do we investigate surfaces? Low energy electrons(Ekin < 1000 eV) are surface sensitive: penetration/escape depths < 100 Å hvin hvout e- Photons in/electrons out: e- Are surface sensitive 16 How do we investigate surfaces? Photon penetration and escape depths, typically > 0.1 microns, not surface sensitive hvin hvout Photons in/photons out: Not surface sensitive 17 Since we are interested in the structures of (typically) the outermost 1-20 atomic layers, we want surface probes with sampling depths of ~ 50 Å or less What determines sampling depth Typically, it is the escape depth of the detected photon/ion/electron Photon escape depths typically ~ 10 nm or more (not good) Ions, can be as little as one monolayer, but may present other problems Electrons ~ escape depth determined by inelastic mean free path (IMFP = λ) Typically, λ = λ(KE, electron density of medium) From surf. Sci. Western (Univ. of W. Ontario) hv Surface, no electrons attenuated e- e- e- Some e- from bulk or near/surface suffer inelastic collisions, change kinetic energy, lose chemical information λ and the continuum model for attenuation of electron intensity Electron intensity out (Ix) Electron intensity in I0 dI = -(dx/λ) I dI/I = -dx/λ I(x) = I0 exp (-x/λ) dx λ-1 is the probability per unit length for the electron to undergo inelastic collision An overlayer thickness of λ will attenuate signal intensity by a factor of 1/e At a thickness of 3λ, signal attenuated by 1/e3 ~ 98% Since we want escape/sampling depths < 100 Å, we want to detect low energy electrons coming from surfaces (EK < 1000 eV) From surf. Sci. Western (Univ. of W. Ontario) Surface Probes using low energy electrons Technique In Out XPS hv ~ 1250 ev-1500ev e-, Ek < 1000 eV AES e- ~ 3000 eV e-, Ek < 1000 eV LEED e- ~ 50-300 eV e-, Ek = Ein UPS hv ~ 21 – 40 ev e-, EK < hv Development of low energy electron-based surface probes: 1. LEED (low energy electron diffraction) –since 1927 (Davisson and Germer and the birth of modern quantum mechanics) 2. AES (Auger electron spectroscopy) –1960’s, Palmberg, et al. 3. XPS (x-ray photoelectron spectroscopy )-1960’s Kai Sigbahn and others at Uppsala University All the above linked to technological developments: LEED: Glass-based vacuum systems, fluorescent screens AES, XPS: Development of accurant electron energy analyzers All the above: improved vacuum technology Improvements: *Angle-Resolved photoemission, (band structure) *spin polarized LEED (magnetic systems) *spin-polarized photoemission (magnetic systems) *time –resolved measurements (has not caught on) *synchrotron-based photoemission, very popular for tuning sampling depths.