VSS FSS TSS TDS TS
advertisement
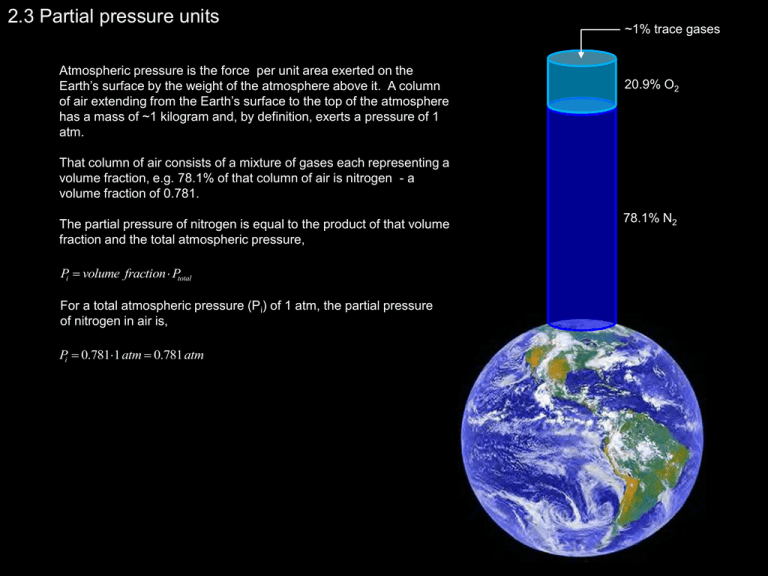
2.3 Partial pressure units Atmospheric pressure is the force per unit area exerted on the Earth’s surface by the weight of the atmosphere above it. A column of air extending from the Earth’s surface to the top of the atmosphere has a mass of ~1 kilogram and, by definition, exerts a pressure of 1 atm. ~1% trace gases 20.9% O2 That column of air consists of a mixture of gases each representing a volume fraction, e.g. 78.1% of that column of air is nitrogen - a volume fraction of 0.781. The partial pressure of nitrogen is equal to the product of that volume fraction and the total atmospheric pressure, Pi volume fraction Ptotal For a total atmospheric pressure (Pi) of 1 atm, the partial pressure of nitrogen in air is, Pi 0.7811 atm 0.781 atm 78.1% N2 2.5 Other types of units Equivalent weight The number of equivalents per mole that a chemical species represents is, for acids, the number of moles of protons that it can donate; and, for bases, the number of moles of H+ that would react with that base. Acids HCl 1H Cl 1 equivalent H 2 SO4 2 H SO42 Bases 3 4 H 3 PO4 3H PO 2 equivalents 3 equivalents NaOH Na OH 2 2 3 CaCO3 Ca CO 3 4 PO 1 equivalent 2 equivalents 3 equivalents The equivalent weight of a substance is defined as its molecular weight divided by the number of equivalents contributed per mole. For CaCO3, Molecular weight of CaCO3 40 12 3 16 100 Equivalents contributed by CaCO3 2 Equivalent weight of CaCO3 50 Thus the gram equivalent weight (gew) of CaCO3 is 50, i.e. there are 50g of CaCO3 per equivalent. Concentration as a common constituent As rainwater passes over bedrock containing limestone and dolomite, calcium (Ca) and magnesium (Mg) carbonates are dissolved and enter the water as calcium (Ca2+) and magnesium (Mg2+) ions. Once in our water supply systems, these ions may precipitate forming bathtub scum and a hard scale which clogs pipes and reduces the efficiency of hot water heaters. The “hardness” of a water is equal to the sum of its divalent ions (e.g. Ca2+, Mg2+, Fe2+, Mn2+, Sr2+). It is cumbersome to seek expression of the five hardness constituents. Instead, these are expressed as a common constituent, traditionally CaCO3, and summed to yield the total hardness. Expression of the constituents as CaCO3 is accomplished by multiplying the concentration of the constituent by the ratio of the gew for CaCO3 to the gew for the constituent, CaCO3 ; MW 100, eq 2, gew 50 Ca 2 ; MW 40, eq 2, gew 20 Mg 2 ; MW 24, eq 2, gew 12 mgCa 2 50 mg 125 as CaCO3 L 20 L mgMg 2 50 mg 25 104 as CaCO3 L 12 L 50 Total Hardness 229 mg as CaCO3 L This is very hard water and would merit treatment for use as a domestic supply. Particle concentrations The total solids content (TS, mg/l) of a water sample may be divided into dissolved (e.g. salts) and suspended (e.g. particulates) fractions. Each of these may be further divided into inorganic and organic fractions. Total suspended solids (TSS, mg/L) represents the concentration, after drying, of the particles retained on a filter. The filter is then ignited and the material remaining (ash) is termed fixed suspend solids (FSS, mg/L) and that lost volatile suspended solids (VSS, mg/L). FSS and VSS represent the inorganic and organic fractions, respectively. Total dissolved solids (TDS, mg/L) are those which pass through a filter and are quantified by drying the sample. http://www.hhpsd.com/whardness.htm TS VSS FSS TSS Several of these fractions have significance in environmental engineering applications: • TDS (saltiness) is important with respect to drinking water and irrigation; • TSS (as turbidity) is used as a standard for safe drinking water consumption; and TDS • VSS (organic matter) provides a measure of a water to consume oxygen.