Roles of in situ surface modification in controlling the growth and
advertisement

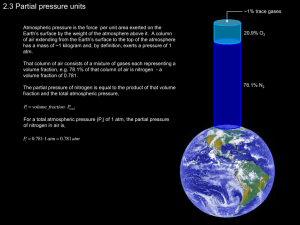
Roles of in situ surface modification in controlling the growth and crystallization of CaCO3 nanoparticles, and their dispersion in polymeric materials Ahmed Barhoum1,2,3*, Luk Van Lokeren1, Hubert Rahier1, Alain Dufresne3,4, Guy Van Assche1 1 Department of Materials and Chemistry, Vrije Universiteit Brussel (VUB), Pleinlaan 2, 1050 Brussels, Belgium 2 SIM vzw, Technologiepark 935,BE-9052 Zwijnaarde, Belgium 3 Université Grenoble Alpes, LGP2, F-38000 Grenoble, France 4 CNRS, LGP2, F-38000 Grenoble, France Corresponding author: ahmed.abdelrasoul@vub.ac.be Supplementary Material 1. Preparation and characterization of calcium carbonate During the preparation of CaCO3, the pH of the slurry varies. The pH of the aqueous CaO slurry is about 13~14. When the CO2 gas is bubbled in the solution, the pH decreases slowly from 12.4 then it stays stable for a comparatively long time (around 45-100 min). After that the pH decreases to 9 in a few minutes. Before bubbling of CO2 gas, CaO particles (Figure S1) dissolve to deliver OH− ions that keep the pH high at 14. When CO2 gas is bubbled in water (Figure S2), the dissolution of CO2 gas in the solution delivers H2CO3 that reacts with OH− ions equilibrating 1 the pH at 12.4. After complete consumption of CaO (end of the reaction) the pH of the system decreases suddenly from 12.4 to 9. Figure S1. SEM image of the commercially CaO powder Figure S2. Schematic representation of the system used in preparing the CaCO3 nanoparticles Phase identification, amorphous content (%) and the average crystallite size of the prepared CaCO3 samples were determined using X–ray diffraction (XRD, Diffractometer Bragg Bantano, Bruker D500, Germany) with Cu-Kα (λ = 1.5406 Å) radiation and a secondary graphite mono chromator. Scanning step size was 0.02°. The following slits were applied: divergence slit 0.3°, receiving slit 0.15° and soller slit 2.3°. The Cu Kα radiation was applied with the tube working conditions 40 kV and 40 mA. Peak position, area and crystallite size were determined from the fitted profiles using FP function (fundamental parameter) with the TOPAS software (Bruker). 2 The average crystallite size was determined according to the Debye Scherrer equation using TOPAS software (Bruker). The amorphous CaCO3 content (crystallintiy %) was calculated by Diffrac. Suite software (Bruker). The average particle size and morphology were examined by a high resolution transmission electron microscopy (HRTEM, Jeol, JEM-2010, Japan). The samples were examined with an acceleration voltage up to 120 kV, a magnification power up to 600 k, and a resolving power down to 0.2 nm. A droplet of CaCO3 water suspension was pipetted onto a wholly carbon–coated TEM grid and allowed to dry before the measurements. The average particle size was measured from HRTEM photos using the specially written software that determined boundaries of each crystal on the photo and measured its area in real units. Raman spectra were recorded in the backscattering configuration on a LabRAM HR Evolution HORIBA spectrometer (1800 grooves/mm) under a confocal microscope with a 50X objectives focusing the 532 nm line. Measurements were performed using laser output power of 2.5 mW, pinhole 1000 µm and slit 100 µm. Data acquisition and spectra treatment were carried out with the commercially available program LabSpec 6 (HORIBA Scientific). For all spectra, background subtraction and peak analysis were performed with the program Origin-lab 7.5 professional combined with an additional peak-fitting module. The X-ray photoelectron spectroscopy (XPS) measurements were performed on a PHI 5600 spectrophotometer with an Al-Kα monochromated source operating in a vacuum of 5x10–9 Torr base pressure was used to identify the core levels of the elements of the synthesized CaCO 3. The samples were irradiated with 187.86 eV, X-ray flux 200 Watt, takeoff angle of 45°. The highresolution Ca(2p), C(1s) and O(1s) core-levels were obtained at a pass energy of 46.5 eV. The outcome electrons were analyzed by a spherical capacitor analyzer using the slit aperture of 0.8 3 mm. Sample charging was compensated by using the charge neutralizer, with an additional mathematical shift relative to the reference (C1s at 285 eV). The spectra acquisition parameters (channel exposition, number of scans, analyzer parameters, etc.) were selected in order to provide the best energy resolution and signal/noise ratio. Peak position, peak intensity, and full width at half–maximum (FWHM) of the samples were calculated using Multipack software (provided by Physical Electronics). The water contact angle (WCA) was measured with a Kruss DSA-100 contact angle analyzer. The measurements were performed on the prepared CaCO3 powders compressed into discs using 5 μL water droplet volume and the contacted angle was determined from the profile of the droplets that were fully separated from the pump syringe needle tip. The discs were prepared by compression under controlled conditions: 100 mg of the sample and a pressure of 107 Pa, in a typical IR die. The zeta potential of CaCO3 particles in suspension was measured at 25°C using a Zeta meter 3.0 equipped with a microprocessor unit (Malvern Instrument Zetasizer 2000). The prepared samples contain 1 mg of CaCO3 dispersed in 100 g monodistilled water. The samples were treated by ultrasonic-horn for 30 min and magnetically stirred for 10 min. The unit automatically calculates the electrophoretic mobility of the particle and converts it into zeta potential using the Smoluchowski equation. Thermogravimetric analysis was performed on a TGA Q5000 (TA Instruments, USA). The samples were dried isothermally at 60 °C for 20 min before heating from 60 to 1000 °C at a heating rate of 10 °C·min–1 under 50 mL·min–1 N2 gas flow. High temperature platinum pans were used and sample mass was approximately 5 mg. The temperature at the maximum rate of decomposition was determined using universal analysis software provided by TA. 4 Thermal degradation of the surfactants on the surface of the prepared modified CaCO3 was performed using differential scanning calorimetry (TA Instruments, Q2920 DSC, USA). Differential scanning calorimetry was used to determine thermal transitions of the synthesized crystals in the temperature range 60–500 ºC. Temperature and enthalpy calibration were performed using an indium standard. Around 0.5 mg of sample was used. Each sample was heated from 60 to 500 °C at a heating rate of 10 K·min–1 under 25 mL·min–1 gas flow. 2. Preparation and characterization of PCL nanocomposite The CaCO3-PCL composites were prepared by melt mixing at 130 °C using a batch-operated lab-scale twin-screw DSM Xplore Micro-Compounder 15 cc (Figure 2s). The CaCO3 dispersion state was characterized by means of scanning electron microscopy (SEM) using a Jeol JSM7000F equipped with a field emission gun and operating at 10 mm working distance and 5 keV electron beam energy. The SEM was performed on the set of samples with a CaCO3 loading of 3 wt%. Prior to SEM, the samples were microtomed at –100 °C to create a perfect plane face using a Leica Ultracut UCT ultra-cryomicrotome with a glass knife. The sample preparation (ultra– cryomicrotome) for SEM is time-consuming and cutting the sample, handling and storage, making the surface of the cross section rough and hence difficult to distinguish the NPs from the rough surface. Thus, the microtomed samples are stored in a bottle contains liquid nitrogen until the samples were characterized by SEM. Thermogravimetric analysis equipment (TA Instruments, TGA Q5000, USA) was employed to examine the thermal degradation of the samples and CaCO3 content. The samples were dried isothermally at 60 °C for 10 min before heating from 60 to 700 °C at a heating rate of 10 K·min–1 under air atmosphere (25 mL·min–1). High temperature platinum pans were used and sample mass was approximately 5 mg. The CaCO3 content in the composite samples was calculated 5 from the mass loss % for filled samples at 550 °C. The temperature of 550 °C is chosen as at that temperature the polymeric part of the nanocomposite samples completely decomposed while the decarbonation of the carbonate did not start yet. Thermal characterization using differential scanning calorimetry (TA Instruments, Q2000 DSC, USA) was performed using a nitrogen-purged 25 mL·min‒1; the instrument is equipped with a refrigerated cooling system (RCS). Around 6 mg of samples were placed in a DSC cell. Each sample was heated from –90 to 90 °C at a heating rate of 10 °C·min–1 under nitrogen atmosphere with a flow rate of 25 mL·min‒1. The crystallization temperature, Tc, was taken as the peak temperature of the crystallization exotherm. Second heating cycles were used to obtain onset, peak temperatures, as well as melting enthalpies. The isothermal crystallization was performed using modulated-temperature differential scanning calorimetry (MTDSC). The selected temperature modulation conditions during quasi-isothermal experiments were amplitude of 0.5 K and a period of 60 s. Measurements were initiated by erasing the thermal history of the samples during one hour at 130 °C. Thereafter, the sample was kept quasi-isothermal at 50 °C using the modulated temperature program during 1000 min to ensure that a steady-state condition (i.e., equal exothermic and endothermic contributions) is attained. This steady-state is attained after the heat flow signal has reached its baseline level, thus after the main crystallization process of the PCL samples. For tensile strength measurements, tensile strength bars (sample dimensions 90×5×1.5 mm3) were prepared using DSM Xplore Micro-Injection Molding Machine 5.5 mL (Figure 3s supplementary information). The granulated compounds were injection-molded into test bars (DIN 53455) on. The flow direction coincided with the longitudinal direction of the bar. The conditions of nozzle temperature 85 °C, injection pressure 4.5 bars, mould temperature 35 °C 6 and cooling time 2 min, were used. The tensile strength measurements carried out by Instron 5900 R at a cross-head speed of 5 mm·min–1. Each sample was measured three times, the data shown being averages over three measurements. All tensile strength measurements were performed 7 days after compression molding to ensure full crystallization of the PCL nanocomposites. Water contact angle (WCA) of the composite polymer was measured with a Kruss DSA-100 contact angle analyzer. The contact angle of water on the substrate was calculated based on a numerical solution of the full Young–Laplace equation by a computer program from the equipment supplier. All contact angle measurements were carried out at 25 °C from the profile of the droplets that were fully separated from the pump syringe needle tip. The droplet volume was 5 µL and at least three parallel measurements were recorded. Figure S3. Batch-operated lab-scale twin-screw DSM Xplore Micro-Compounder 7 Figure S4. DSM Xplore Micro-Injection Molding 5 mL 8 3. Results Figure S5. XPS spectra of pure CTAB and sodium oleate Figure S6. Thermogram of pure CTAB and sodium oleate; (a) TGA mass loss curve; (b) DTG derivative mass loss; (c) DSC curve 9 Figure S7. Derivative weight curves by TGA of pure PCL (BK) and PCL loaded with the prepared CaCO3 fillers 10 Figure S8. Data as calculated from TGA and DSC curves of pure PCL (BK) and CaCO3-PCL composites; (a) TGA maximum decomposition temperature; (b) DSC melting temperature at peak maximum; (c) DSC melting enthalpy; (d) DSC crystallization enthalpy 11 Table S1. XPS data of the prepared calcium carbonates Calcium carbonate core levels Sample Ca 2p C 1s O 1s Ca 2p1/2 Ca 2p2/3 CO3 *CxHy Peak position (eV) MC 347.0 350.7 289.7 285 531.5 NC 346.9 350.6 289.5 285 531.5 CC 346.8 350.5 289.4 285 531.3 OC 346.7 350.4 289.4 285 531.2 Peak relative intensity (%) MC 45.20 19.4 12.60 12.60 100 NC 38.22 14.52 11.11 10.68 100 CC 39.1 15.38 11.5 12.67 100 OC 43.2 17.69 12.9 21.7 100 Atomic concentration (%) MC 16 36.2 47.8 NC 13.3 33.4 53.3 CC 14.7 35.1 50.2 OC 14.3 43.3 42.3 * C 1s (CxHy) is due to carbon of surfactant and carbon of contamination from the environment i.e. CO2 12 Table S2. TGA data of the prepared calcium carbonates Sample Temperature range Temperature range 100 ºC – 500 ºC 500 ºC – 1000 ºC compound W1 [%] Td [°C] TR [°C] W2 [%] MC H2 O 1.7 752 183 41.3 NC H2 O 1.9 745 199 41.5 CC H2O + CTAB 2.2 729 210 41.6 OC H2O + oleate 3.3 712 228 41.9 W1, mass change due to loss of crystalline water and surfactant; Td, decomposition temperature of decarbonation and losing CO2; TR, decomposition temperature interval of decarbonation process; W2, mass change due to loss of CO2 Table S3. DSC data of the prepared calcium carbonates decomposition enthalpy Sample [°C] [J.g-1] MC 356 8 NC 330 20 CC 325 99 OC 361 311 13 Table S4. Thermo-mechanical properties of pure PCL and the prepared CaCO3-PCL NCs Sample Pure PCL PCL filled MC PCL filled NC PCL filled CC PCL filled OC content Td To Tm Hm Tc Hc [wt%] [ºC] [ºC] [ºC] [J.g-1] [ºC] [J.g-1] E [Mpa] 0.00 328 294 56.5 67.7 35.4 64.5 345 0.70 333 303 56.9 69.0 35.9 64.0 349 2.50 346 313 56.7 69.3 36.2 64.0 417 4.40 335 318 56.9 68.6 35.9 63.1 458 5.40 323 303 56.6 67.4 36.2 63.0 485 0.60 337 303 56.7 69.3 35.5 63.1 437 2.70 324 296 56.6 69.5 35.7 63.3 379 4.20 326 294 56.5 68.1 36.1 63.1 424 5.50 312 288 56.4 68.8 36.2 63.9 436 0.60 353 335 56.8 68.7 35.4 62.9 432 2.40 357 332 56.8 69.9 36.0 63.4 433 4.20 339 325 56.5 68.9 35.8 63.1 451 5.70 332 317 57.0 67.2 35.9 63.3 474 0.70 342 315 56.8 68.4 36.0 64.1 397 2.20 330 308 56.7 68.9 36.1 64.3 409 4.10 393 292 56.4 70.4 36.0 64.2 421 5.80 326 283 56.6 69.8 36.5 64.7 457 To, onset decomposition temperature; Td, decomposition temperature at inflection point; Tm, melting temperature at inflection point; Hm, melting enthalpy; Tc, onset crystallization temperature; Hc, crystallization enthalpy; E, elastic modulus 14