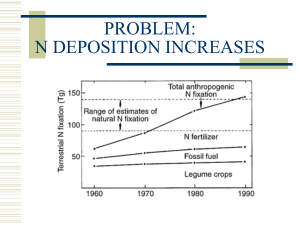
- Lorentz Center
advertisement
HELIOS Nanostructured Water Oxidation Photocatalysts Heinz Frei February 3, 2010 Goal: CO2 + H2O CH3OH + O2 visible light • Conversion in a single integrated system (terawatt scale) hv O2 H2O CH3OH CO2 reduction CO2 H2O O 2 H2O oxidation • Inorganic system robust Topics today: Robust inorganic nanoclusters as water oxidation catalysts All inorganic photocatalytic units in nanoporous silica scaffolds Turnover frequencies (TOF) for oxygen evolution at Co and Mn oxide materials reported in the literature Oxide TOF (sec-1) Overvoltage, η (mV) pH T (oC) Quantum yield Reference Co3O4 0.035 325 5 RT 58% Harriman (1988) [1] Co3O4 > 0.0025 350 14 30 -- Tamura (1981) [2] Co3O4 > 0.020 295 14 120 -- Wendt (1994) [3] Co3O4 > 0.0008 414 14.7 25 -- Tseung (1983) [4] Co3O4 > 0.006 235 14 25 -- Singh (2007) [5] Co,P film > 0.0007 ~ 0.1 410 7 7 25 60 --- Nocera (2008) [6] Nocera (2009) [7] MnO2 > 0.013 440 7 30 -- Tamura (1977) [8] Mn2O3 0.055 325 5 RT 35% Harriman (1988) [1] [1] [2] [3] [4] [5] [6] [7] [8] Harriman, A.; Pickering, I.J.; Thomas, J.M.; Christensen, P.A. J. Chem. Soc., Farad. Trans. 1 1988, 84, 2795-2806. Iwakura, C.; Honji, A.; Tamura, H. Electrochim. Acta 1981, 26, 1319-1326. Schmidt, T.; Wendt, H. Electrochim. Acta 1994, 39, 1763-1767. Rasiyah, P.; Tseung, A.C.C. J. Electrochem. Soc. 1983, 130, 365-368. Singh, R.N.; Mishra, D.; Anindita; Sinha, A.S.K.; Singh, A. Electrochem. Commun. 2007, 9, 1369-1373. Kanan, M.W.; Nocera, D.G. Science 2008, 321, 1072-1075. Nocera, D.G. Symposium Solar to Fuels and Back Again, Imperial College, London, 2009. Morita, M.; Iwakura, C.; Tamura, H. Electrochim. Acta 1977, 22, 325-328. Nanostructured Co oxide cluster in mesoporous silica scaffold Synthesis of Co oxide clusters in SBA-15 using wet impregnation method 35 nm bundles (4 % loading) 65 nm bundles (8 % loading) XRD SBA-15/Co3O4 (8%) SBA-15/Co3O4 (4%) Co3O4 EXAFS free nanorod bundle Co3O4 bulk SBA-15/Co3O4 (8%) SBA-15/Co3O4 (4%) • Co oxide clusters are 35 nm bundles of parallel nanorods (8 nm diameter) interconnected by short bridges • XRD, Co K-edge EXAFS and reveal spinel structure Co L-edge XAS spectrum • Co L-edge absorption spectrum confirms Co3O4 structure Efficient oxygen evolution at Co3O4 nanoclusters in mesoporous silica SBA-15 in aqueous suspension Mass spectroscopic monitoring O2 evolution SBA-15/Co3O4 35 nm bundle TOF 1140 s-1 per cluster 65 nm bundle O2 Co3O4micron sized particles SBA-15/NiO (8%) F. Jiao, H. Frei, Angew. Chem. Int. Ed. 49, 1841 (2009) • Visible light water oxidation in aqueous SBA-15/Co3O4 suspension using Ru2+(bpy)3 + S2O82- method. Mild conditions: 22oC, pH 5.8, overvoltage 350 mV • High catalytic turnover frequency: 1140 O2 molecules per second per cluster TOF of catalyst per projected area = 1 s-1nm-2 mesoporous silica membrane, 150 μ thick: TOF = 100 s-1nm-2 • O2 yield is 1600 times larger than for 35 nm bundle catalyst compared to μ-sized Co3O4 • Surface area of nanorod bundle cluster = factor of 100, catalytic efficiency of surface Co centers = factor of 16 Co K-edge: No sign of Co oxidation state change after photolysis EXAFS: No sign of structural change after photolysis • Co3O4 structure in silica scaffold stable under water oxidation catalysis • Rate and size of the SBA-15/Co3O4 catalyst driven by visible light are comparable to Nature’s Photosystem II and are in a range that is adequate for the keeping up with solar flux (1000 W m-2) TOF 300 s-1 TOF 1140 s-1 • Abundance of the Co metal oxide, stability of the nanoclusters under use, modest overpotential and mild pH and temperature make this a promising catalyst for use in integrated artificial solar fuel systems Efficient oxygen evolution at nanostructured Mn oxide clusters supported on mesoporous silica KIT-6 TEM XAFS MnO1.51 calcined 600 oC KIT-6 (3D channels) MnO2 Mn2O3 Mn3O4 400 oC 64% 36% - 500 oC 95% 5% - 600 oC 6% 80% 14% 700 oC - 81% 19% 800 oC - 70% 30% 900 oC - 51% 49% • Spherical Mn oxide nanoclusters, 70-90 nm diameter, mixed phase (calcination T) • The phase composition was determined by component analysis of XANES spectra Efficient oxygen evolution in aqueous solution using Ru2+(bpy)3persulfate visible light sensitization system Mass Spec s-1 Mild conditions: pH 5.8, 22 oC overvoltage 350 mV TOF 3,320 per cluster O2 evolution TOF 900 s-1 per cluster F. Jiao, H. Frei, submitted • Most active catalyst: MnO1.51 with TOF = 3,320 O2 s-1, which corresponds to 0.6 sec-1 nm-2 projected area 200 μm membrane with TOF of 100 s-1nm-2 meets solar flux • Very stable upon photochemical use, no leaching of Mn • Silica scaffold provides: • high, stable dispersion of nanostructured catalysts • sustained catalytic activity by protecting the active Mn centers from deactivation by surface restructuring Co or Mn oxide/ silica core shell constructs Mn oxide core/ silica shell construct silica shell Co3O4 or MnOx core Reverse microemulsion method (Ying, J.Y., Langmuir 24, 5842 (2008)) F. Jiao Precise matching of redox potentials of the components in organic molecular systems Hammarstrom, Chem. Soc. Rev. 30, 36 (2001) Approach: Well-defined all-inorganic polynuclear photocatalysts arranged in robust 3-D nanoporous scaffold nanoporous silica support 200 nm • Photocatalytic site consists of a hetero-binuclear unit acting as visible light charge transfer pump driving a multi-electron transfer catalyst • 3-D nanoporous support for arranging and coupling photoactive units • High surface area required to avoid wasting of solar photons (one photocatalytic site nm-2 assuming rate of 100 sec-1) • Nanostructured support for achieving separation of redox products MCM-41 SBA-15 Selective assembly of binuclear MMCT units for driving water oxidation catalysts: TiOCrIII h DRS e- MMCT (visible light) O CrIII 1-R Ti 0.9 OO O O O O Si Si Si Al Si Si 0.6 TiIV-O-CrIII TiIII-O-CrIV 0.3 0.0 CrVI(=O) + TiIII CrV-O-TiIV Selective redox coupling CrV EPR 3 10 Cr-AlMCM-41, cal 630C as-syn TiCr-AlMCM-41 1 0 400 600 800 Wavelength (nm) 6000 6040 Energy (eV) 2.00Å(CrIII-O) 6 2.70Å(CrIII-O-Ti) 4 1.59Å(CrVI=O) TiCr-AlMCM-41 2 Cr-AlMCM-41 0 0 1 2 3 4 5 6 Distance R (Å) 7 Relative intensity 2 TiCr-AlMCM-41 EXAFS FT Magnitude Normalized Absorption X-ray K-edge III Cr -AlMCM-41 0.5 0.0 V Cr Sp: g=1.977, g//=1.96 TiCrAl-MCM41 VI Cr Al-MCM41 -0.5 3200 3300 3400 3500 Magnetic field (G) Han, Frei, J. Phys. Chem. C 112, 8391 (2008) • Cr EPR, XAFS K-edge, EXAFS, FT-Raman and optical spectroscopy allows step-by-step monitoring of oxidation state and coordination geometry changes of the Cr center upon TiOCr formation Selective assembly of binuclear MMCT units for driving water oxidation catalysts: TiOCrIII Cr-O CrIII TiOCrIII Cr-O B -4) |(R)| (Å |(R)| (Å -4) 8 4 B 8 4 Cr--Ti 0 0 2 4 R (Å) 6 0 8 0 2 4 R (Å) 6 8 Cr EXAFS curve fitting: Cr-O N DW Cr-O N DW 1.97 A 3.8 0.003 2.01 A 3 0.001 3.14 1.72 A 1 0.003 • Second Cr---Ti N DW 1 0.007 Cr----Si N DW 2.89 3 0.003 shell peaks confirm oxo bridge structure of MMCT unit • Cr-O bond of Ti-O-Cr bridge is shorter than for Cr-O-Si, indicating partial charge transfer character of ground state Binuclear TiOCrIII pump drives H2O oxidation catalyst under visible light 10 nm HR-TEM of Ir oxide nanoclusters in silica channels O2 evolution using Clark electrode Quantum yield = 14% (lower limit!) O2(mg/L) 9 Level of saturated O2 in water 6 Light on 3 IrxOy-TiCr-AlMCM-41 0 -0.5 0.0 0.5 1.0 1.5 2.0 Time (hour) Han, Frei, J. Phys. Chem. C 112, 16156 (2008) Nakamura, Frei, J. Am. Chem. Soc. 128, 10689 (2006) • Efficient visible light water oxidation in aqueous suspension observed EPR and FT-Raman spectroscopy show formation of TiIV…O2- complex MMCT Ti -O-Cr /IrOx hv IV TiIV…O2- g2 = 2.010 g1 = 2.034 1.0 1.0 simulated spectrum 0.5 after photolysis superoxide 0.0 g3= 2.005 Raman intensity (a.u.) 3200 before photolysis 3250 3300 Magnetic field (G) 961 0.0005 0.5 0.0 3200 16O18O18O 2 TiIII-O-CrIV/IrOx TiIII EPR Intensity Relative intensity 10 3 III III superimposed EPR spectrum of simulate Ti and Cr photolysis of IrxOy-TiCr-AlMCM-41+H2O photolysis of IrxOy-Cr-AlMCM-41+H2O 3300 3400 3500 Magnetic field (G) FT-Raman 930 18 after photolysis in H2 O 994 O216 after photolysis in H2 O O2 trapped by transient TiIII O2- detected in aqueous solution 18O labeling of superoxide when using H218O 1100 1000 900 -1 Raman shift (cm ) • Electron donation from IrOx catalyst competes successfully with back electron transfer from TiIII • Flexibility of donor metal selection for matching redox potential of charge-transfer chromophore and catalyst V Elucidation of electron transfer pathways and kinetics of binuclear charge-transfer chromophore by transient absorption spectroscopy L-edge X-ray absorption DRS Ti TiMnII-MCM-41 MnII • • Transient absorption spectroscopy of MMCT units using indexmatching liquids (mineral oil, silicone oil, or CHCl3) 5 nanosecond resolution Transient bleach of MMCT transition observed Excitation of TiOMn, 400-600 nm Pump Dependence: Kinetic and Spectral (a) (b) -3 -2 OD (10 )(t=0, tavg) -3 OD (10 ) 0 -4 TiMn-SBA-15 -6 -8 0 2 4 6 Time (s) Pump Spectral Dependence: DRS Comparison Probe: 400nm Pump 425 nm 445 nm 475 nm 535 nm 8 10 Albery model for dispersive 1st order kinetics: (Albery et al., J. Am. Chem. Soc. 1985, 107, 1854) k = k’exp(γx), Gaussian distribution in ln(k) mean time constant 1/k’ = 1.8 μsec 0 1/k' = 1.8 ± 0.3s = 2 ± 0.2 -5 -10 t=0 Albery Fits, normalized data tavg, unnormalized data DRS Static Spectra 400 450 500 550 Pump (nm) 600 T. Cuk, W. Weare, H. Frei, J. Phys. Chem. C, submitted • Recovering bleach is due to back electron transfer of excited TiIIIOMnIII → TiIVOMnII • Spread of first order rate constants indicates structural heterogeneity of the silica environment of the binuclear sites Unusually slow back electron transfer Ti(III)OMn(III) e1(Ti)t2g3(Mn)eg1(Mn) S= 5/2 Ti(IV)OMn(II) S = 3/2 hv MMCT G e0(Ti)t2g3(Mn)eg2(Mn) S= 5/2 • Substantial structural rearrangement of coordination sphere in excited MMCT state and polarization of the silica environment imposes barrier to back electron transfer • Lifetime long → MMCT units suitable for driving MET catalysts with visible light Selective assembly of binuclear MMCT units for driving water oxidation catalysts: TiOCoII, TiOCeIII O Ti O O O O 0.6 O Si Si Si Si O Si Si DRS 0.4 1-R Ti-MCM-41 Ce-MCM-41 0.2 MMCT 300 400 TiIV-O-CeIII TiIII-O-CeIV 500 600 700 Normalized Absorption XAFS TiCe-MCM-41 0.0 Han, Frei, J. Phys. Chem C 112, 8391 (2008); Microporous Mesoporous Mater. 103, 265 (2007) Nakamura, J. Am. Chem. Soc. 129, 9596 (2007) 5728 TiCeIII 5727 3 E = +1 eV CeIII 1 0 Wavelength (nm) 0.4 1-R Co-MCM-41 0.2 MMCT 0.0 TiIII-O-CoIII Relative Intensity 3 TiIV-O-CoII b a 5730 5760 Energy (eV) TiCo-MCM-41 Ti-MCM-41 + Co-MCM-41 A 4 2 Normalized Absorption Si III Ce 10 1.5 g = 5.250 CoII Ce L-edge 2.4 5729 5737 b' 1.6 a' 0.8 CeIV 0.0 5720 5760 Energy (eV) 800 5800 EPR g// = 2.032 1.0 b 0.5 g = 5.107 g// = 2.034 a CoII linked to Ti is high spin, tetrahedral 0.0 400 600 Wavelength (nm) B TiCeIV 2000 3000 4000 Magnetic Field (G) • Selective assembly due to higher acidity of TiOH vs. SiOH • MMCT excitation by visible light generates donor centers (CeIV, CoIII) of sufficiently positive potential for driving H2O oxidation catalyst Coupling polynuclear photocatalysts in nanoporous silica scaffolds to achieve separation of reduced products from evolving oxygen Long term goal: CO2 + H2O CH3OH + O2 visible light hν O2 H2O CH3OH (L) (L = inorg. or C-based conducting linker) CO2 CO2 reduction Two photon system H2O O2 H2O oxidation envisioned integrated system • Coupling of fuel generating photocatalytic sites (green) with O2 evolving sites (purple) across nanoscale wall • Separation of oxygen from methanol Co or Mn oxide/ silica core shell constructs with nanowires penetrating SiO2 shell Mn oxide core/ silica shell construct silica shell Co3O4 or MnOx core Reverse microemulsion method (Ying, J.Y., Langmuir 24, 5842 (2008)) F. Jiao HELIOS Conclusions • Development of all-inorganic photocatalytic units on nanoporous silica supports consisting of heterobinuclear charge-transfer chromophore coupled to multi-electron catalyst; selective, flexible synthetic methods (abundant elements, scalable synthetic approach) • MMCT chromophores absorb deep in the visible region, possess donor and acceptor centers with selectable potentials → key to thermodynamic efficiency of photocatalyst • Long lifetime (microsec) of MMCT states uncovered • H2O oxidation to O2 under visible light (TiOCrIII chromophore driving an IrOx nanocluster catalyst) at > 14 % quantum efficiency, hydroperoxide intermediate observed • Co3O4 and MnO1.51 nanocluster catalysts of abundant materials for water oxidation, TOF in range suitable for keeping up with solar flux HELIOS Acknowledgments Postdoctoral Fellows: Feng Jiao Walter Weare Hongxian Han Tania Cuk (Miller fellowship) N. Sivasankar Marisa MacNaughtan Drs. Vittal Yachandra, Junko Yano Facilities: NCEM-LBNL, SSRL US Department of Energy, Office of Basic Energy Sciences, Division of Chemical, Geological and Biosciences Helios Solar Energy Research Center, funded by DOE-BES