MS PowerPoint

Mesostructured Hollow Spheres of Graphitic N-Doped
Carbon Nanocast from Spherical Mesoporous Silica
J. Phys. Chem. B 2004 , 108, 19293- 19298
Introduction
Porous carbon - high chemical stability, large surface area and tailorable structure property.
High SA and meso str. – more dispersion of metal ions on their surface
it is difficult to achieve highly dispersed metal catalyst due to the inert surface of the carbon materials
Generation functional groups on the carbon surface – by harsh conditions
Functional groups by in situ generation – N-carbon source
Varying carbonization temperature – change in N/C ratio
by adopting CVD method N- OMC were prepared
(SBA-15 hard template, acetonitrile carbon source)
SBA-15
4g P123 + 0.5 g CTAB + 25 ml EtOH + 30 ml H
2
O + 60 ml HCl (2M)
Stirred at RT
10 g TEOS
Stirred at RT for 1 h
Autoclave at 80 0 C for 6 h
Autoclave at 110 0 C for 12 h
Washed EtOH and dried Cal at 550 0 C
SBA-15
NOMCs (CNx)
0.5 g SBA-15
N
2
& sat. with acetonitrile
Carbonized at 900-1000 0 C for 3 h under N
2 atm.
Washed with 20 % HF at RT and with EtOH
NOMCs
N
2sorption isotherm & SEM of SBA-15
BET
PD = 6.0 nm
P vol
SA
=1087 m 2 /g
= 1.13 cc/g
SEM & TEM images OMC
(f)
SEM images of OMCs compaction at 1.0 Gpa for 1 h.
1000 0 C 1000 0 C
900 0 C 900 0 C
XRD of OMC
2θ = 2.11 ° - d
200 basal d
100
= 8.4 nm & a
0
= 9.7 nm
2θ = 26.2 ° - d
002
= 0.339 nm
N
2sorption isotherm & TEM of OMC
BET
PD = 4.7 nm
P vol
SA
=779 m 2 /g
= 0.66 cc/g
Mechanism for the Formation of Mesoporous Carbon Hollow Spheres
Textural properties
XPS of OMC
N1s C1s
N1s
400.8eV – quaternary N atoms
398.9 eV – pyridine - like N atoms
C1s
284.6 eV – sp 2 gC species
Raman spectra of OMC
Conclusion hollow spheres of structurally wellordered – NOMC may be nanocast using SBA-15 template via a CVD route
The CVD temperature is an important consideration and should be higher than 900 ° C and preferably 1000 ° C for successful formation of carbon hollow spheres.
The use of acetonitrile as a carbon precursor results in N-doped (CNx) materials with a nitrogen content of ca. 6.5 wt %.
The CNx hollow spheres exhibit a high level of graphitization especially for materials prepared at a CVD temperature of 1000 ° C
Preparation of
Pt/CMK-3
Anode Catalyst for Methanol
Fuel Cells Using Paraformaldehyde as Reducing Agent
Chinese J Catal, 2007, 28(1): 17-21
Introduction
In recent years, DMFC have attracted significant attention because of their high energy transfer efficiency and low pollution causing potential.
The disadvantages of the existing catalyst low catalytic activity
The highest electro catalytic activity of two component system is Ru-Pt/C
In this study Pt/CMK-3 anode catalyst for DMFC was prepared by a novel liquid reduction method using paraformaldehyde has reducing agent.
SBA-15
4g P123 + 22 ml H
2
O +38 ml HCl (2M)
Stirred at 35 0 C
7 g TEOS
Stirred at 35 0 C for
24 h
Aged at 100 0 C for 48 h
Washed EtOH and dried Cal at 550 0 C
SBA-15
Msoporus Carbon
1 g SBA-15 + 1.25 g sucrose + 0.14 g H
2
SO
4
+ 5 g H
2
O
Dried at 100 0 C for 6 h and 160 0 C for 6 h
Black powder
0.8 g sucrose + 0.09 g H
2
SO
4
+ 5 g H
2
O
Dried at 100 0 C for 6 h and 160 0 C for 6 h
Carbonized at 900 0 C for 6 h under N
2 atm.
Washed with 5% HF at RT and with EtOH
CMK-3
Preparation of Pt/CMK-3
60 mg CMK-3 + 20 ml H
2
O +0.039 M H
2
PtCl
6
Ultrasonication 30 min
Heated at 343 K / N
2
10 ml Na
2
CO
3
+ Paraformaldehyde
Stirred 2.5 h
Filt. Washed & dried
Pt/CMK-3, Pt/C-M
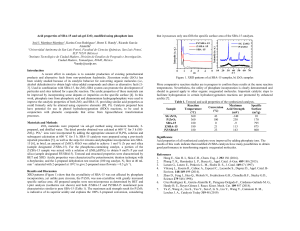
XRD patterns of the Pt/CMK-3, Pt/C-M and Pt/XC-72
Pt/C-M
Pt/XC-72
Pt/CMK-3
Average diameter and relative crystallinity of Pt particles
TEM images of SBA-15 and CMK-3
TEM images of Pt/CMK-3 and Pt/XC-72
Particle size distribution
Pt/CMK-3 Pt/XC-72
The average size of the Pt particles in the Pt/CMK-3 catalyst is 2.8 nm
The average particle size Pt/XC-72 is 3.5 nm
Particles aggregation is observed in Pt/XC-72 more then 10 nm
Electrocatalytic activity of the catalysts for methanol oxidation
8 mg Pt/CMK-3 + 0.6 ml EtOH +2.4 ml H
2
0.2 ml Nafion (5 %)
O +
Sonication 30 min
4 µl slurry on GC electrode
Dried at 308 K
0.5 M H
2
SO
4
+ 0.5 M MeOH
N
2 bubbled to remove O
2
50 mV/s at 309 K
Cyclic voltammograms of Pt/CMK-3, Pt/C-M and Pt/XC-72
1.Pt/C-M
2. Pt/XC-72
3.Pt/CMK-3
Positive scan, the potential of oxidation peaks of CH
0.64 V
3
OH for all 3 catalyst at
Negative scan the potential located at 0.43 V
Methanol is dissociatively adsorbed on the Pt surface
Increasing potential platinum oxides are formed and reaction rate is enhanced
Current density is increased
The electrocatalytic activity is related to the size of Pt particles. Smaller the particle size the activity is relatively higher
Catalyst
Pt/CMK-3
Pt/XC-72
Pt/C-M
Peak current
(mA cm -2 )
22.06
19.72
14.9
Chronoamperometric Curves
1.Pt/C-M
2. Pt/XC-72
3.Pt/CMK-3
Pt/CMK-3 high catalytic activity
The relative crystalinity and average Pt particle size in
Pt/CMK-3 are lower and smaller.
Catalyst preparation and reduction rate is important factor for the structure of metal deposition.
Creation of metal atoms from chemical reaction
Formation of crystal cells from atoms
Aggregation of cells to from particles
Coalescence of particles
The primary role of carbon support is to disperse metal nanoparticles and provide electrical connection among them.
Conclusion
The nucleation and growth of Pt particles on CMK-3 are influenced by the experimental conditions such as surface states of carbon, temperature, time, and concentration of reactants and products.
Small and uniformly distributed Pt particles can be obtained by controlling the experimental conditions
This preparation method is very simple and the electro catalytic activity of the prepared Pt/CMK-3 catalyst for the methanol oxidation is high, this method is promising for practical applications.