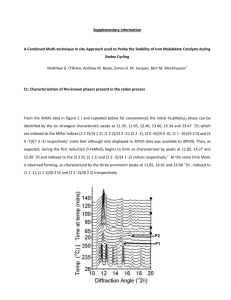
The Busch Catalyst
advertisement
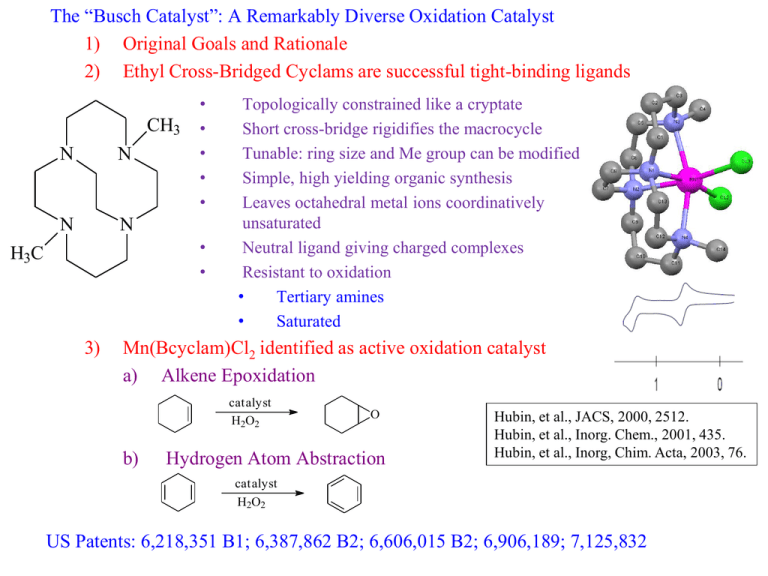
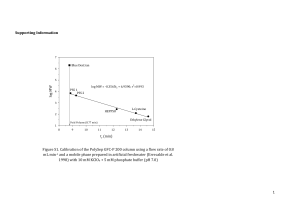
The “Busch Catalyst”: A Remarkably Diverse Oxidation Catalyst 1) Original Goals and Rationale 2) Ethyl Cross-Bridged Cyclams are successful tight-binding ligands CH3 N N N N • • • • • • • H3C 3) Topologically constrained like a cryptate Short cross-bridge rigidifies the macrocycle Tunable: ring size and Me group can be modified Simple, high yielding organic synthesis Leaves octahedral metal ions coordinatively unsaturated Neutral ligand giving charged complexes Resistant to oxidation • Tertiary amines • Saturated Mn(Bcyclam)Cl2 identified as active oxidation catalyst a) Alkene Epoxidation catalyst H2O2 b) O Hydrogen Atom Abstraction Hubin, et al., JACS, 2000, 2512. Hubin, et al., Inorg. Chem., 2001, 435. Hubin, et al., Inorg, Chim. Acta, 2003, 76. catalyst H2O2 US Patents: 6,218,351 B1; 6,387,862 B2; 6,606,015 B2; 6,906,189; 7,125,832 4) Synthesis of the Mn(Bcyclam)(OH)22+ complex (activated form of the catalyst) H2O, H2O2, NH4PF6 Yin, et al., Inorg. Chem., 2006, 8052. 5) Changing the pH of the aqueous solution changes the major species present Shi, et al., Angew. Chem. Int. Ed., 2011, 7321. Yin, et al., JACS, 2007, 1512. 6) Electron Transfer Mechanism a) At pH = 1.5, [LMnIV(H2O)(OH)]3+ is the major species (MnIV—OH for short) b) Oxidation of Ph3P: to Ph3P=O is a commonly studied reaction Xu, et al., Chem. Eur. J., 2009, 11478. 7) Concerted Oxygen Transfer a) Oxidation of Ph3P: at pH = 13.4 [LMnIV(=O)2]o (MnIV=O for short) Xu, et al., Chem. Eur. J., 2009, 11478. 8) Hydrogen Atom Abstraction Mechanism a) Similar to phosphine oxidation, low pH (MnIV—OH) and high pH (MnIV=O) are active catalysts b) c) 9,10-Dihydroanthracene is a common substrate; oxidizes to anthracene Two successive H-atom abstractions occur; the first step is rate limiting Yin, et al., JACS, 2008, 16245. 9) The Oxygen Rebound Mechanism a) Suspected mechanism of oxidation by Cytochrome P450 Enzymes, which protect organisms against toxic organic compounds b) Hydroxylation of Hydrocarbons by High-Valent Metal ions c) MnIV(Bcyclam) is capable of Oxygen Rebound Oxidations as well d) MnIV=O e) i) Is capable of Oxygen Rebound to produce hydroxylated products ii) Does not follow the Electron Transfer Mechanism MnIV—OH i) Is an efficient Electron Transfer catalyst ii) Is incapable of Oxygen Rebound Shi, et al., Angew. Chem. Int. Ed., 2011, 7321. 10) Epoxidation of Alkenes by a Concerted Oxygen Transfer from the Hydrogen Peroxide Adduct a) Organic Peracids (like MCPBA) can epoxidize alkenes b) “Inorganic Peracids” are known to react similarly (Acc. Chem. Res. 2004, 646) c) MnIV(Bcyclam), epoxidizes alkenes in the presence of H2O2 by this mech. Yin, et al., JACS, 2005, 17170. Yin, et al., Inorg. Chem., 2006, 3467. 11) Que has recently studied Fe(Bcyclam) as a catalyst for epoxidation and cis-dihydroxylation of alkenes (Feng, et al., ACS Catalysis, 2011, 1035.) a) Naphthalene 1,2-dioxegenase cis-dihydroxylates aromatic groups b) Que wanted to determine if two available cis sites on the metal were required Fe(Bcyclam)Cl2 + AgOTf c) Tested against Fe(TMC) (tetramethylcyclam) due to cis/trans only difference d) e) f) g) Fe(Bcyclam) was a much better catalyst than Fe(TMC) Mechanistic investigation showed activation with Acetic Acid, but loss of dihydroxylation and only epoxidation Without Acetic Acid, dihydroxylation is favored Que suggests an FeV=O active catalyst for both Feng, et al., ACS Catalysis, 2011, 1035.) Supplementary information from Busch 2006 Inorg. Chem. p8052