File
advertisement

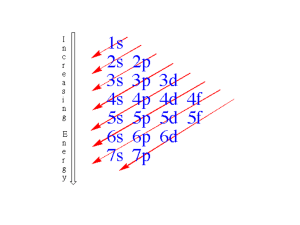
AP Chemistry Unit 3 - Elements Lesson 10 – Electron Configurations Book Section: 6.4-6.9 Electron Configurations This shows the distribution of all electrons in an atom. Each component consists of A number denoting the energy level; Electron Configurations This shows the distribution of all electrons in an atom. Each component consists of A number denoting the energy level, A letter denoting the type of orbital, Electron Configurations This shows the distribution of all electrons in an atom. Each component consists of A number denoting the energy level, A letter denoting the type of orbital, A superscript denoting the number of electrons in those orbitals. Aufbau Principle The order in which orbitals are filled with electrons is known as the Aufbau principle. Full vs. Noble Gas Configuration You can use a noble gas shell to shorten electron configurations. Ex: Sodium 1s22s22p63s1 [Ne]3s1 Periodic Table We fill orbitals in increasing order of energy. Different blocks on the periodic table (shaded in different colors here) correspond to different types of orbitals. Periodic Table Some irregularities occur when there are enough electrons to halffill s and d orbitals on a given row. Periodic Table For instance, the electron configuration for chromium is [Ar]4s13d5 rather than the expected [Ar]4s23d4. Copper is [Ar]4s13d10. Ionizing Transition Metals When transition metals become ions, (Ex – Mn2+), the electrons are lost first from the s block, rather than the d block. Electron configuration for Mn: [Ar]4s23d5 Electron configuration for Mn+2: [Ar]3d5 This is because the orbitals become “rearranged” once the electrons are put in them. (Crystal Field Theory & molecular orbitals – inorganic chemistry in college) AP 1989 MC #4-7 1s22s22p53s23p5 B) 1s22s22p63s23p6 C) 1s22s22p62d103s23p6 D) 1s22s22p63s23p63d5 E) 1s22s22p63s23p63d34s2 4) An impossible electron configuration 5) The ground-state configuration for the atoms of a transition element 6) The ground-state configuration of a negative ion of a halogen 7) The ground-state configuration of a common ion of an alkaline earth element A) AP 1989 MC #4-7 1s22s22p53s23p5 B) 1s22s22p63s23p6 C) 1s22s22p62d103s23p6 D) 1s22s22p63s23p63d5 E) 1s22s22p63s23p63d34s2 4) An impossible electron configuration – C (79% correct) - easy 5) The ground-state configuration for the atoms of a transition element – E (42% correct) - medium 6) The ground-state configuration of a negative ion of a halogen – B (62% correct) - easy 7) The ground-state configuration of a common ion of an alkaline earth element – B (40% correct) - medium A) AP 1984 MC #22 1s22s22p63s23p3 Atoms of an element, X, have the electronic configuration shown above. The compound most likely formed with magnesium, Mg, is MgX Mg2X MgX2 MgX3 Mg3X2 AP 1984 MC #22 1s22s22p63s23p3 Atoms of an element, X, have the electronic configuration shown above. The compound most likely formed with magnesium, Mg, is MgX Mg2X MgX2 MgX3 Mg3X2 - 80% correct, very easy AP 1984 MC #58 Which of the following represents the ground state electron configuration for the Mn3+ ion? (Atomic number Mn = 25) 1s22s22p63s23p63d4 1s22s22p63s23p63d54s2 1s22s22p63s23p63d24s2 1s22s22p63s23p63d84s2 1s22s22p63s23p63d34s1 AP 1984 MC #58 Which of the following represents the ground state electron configuration for the Mn3+ ion? (Atomic number Mn = 25) 1s22s22p63s23p63d4 – 32% correct - hard 1s22s22p63s23p63d54s2 1s22s22p63s23p63d24s2 1s22s22p63s23p63d84s2 1s22s22p63s23p63d34s1 HW: 6.48, 50, 52, 54, 56, 58 This Week: Thursday – Gravimetric Analysis of a Chloride Salt, Quantitative Analysis of Soluble Sulfate Due Friday – Electron Configurations (6.4-6.9) 10/18 – Gravimetric Analysis of a Chloride Salt Due 10/20 – Elements Exam 10/21 – Problem Set 2 Due