powerpoint
advertisement
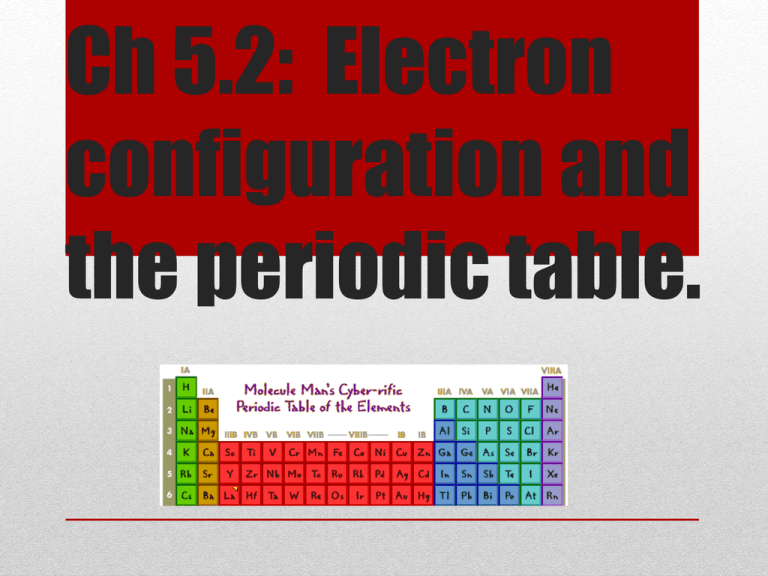
Ch 5.2: Electron
configuration and
the periodic table.
In general terms, the electron
configuration of an atom’s highest
occupied energy level determines the
atom’s chemical properties.
Elements are arranged vertically in groups
that share similar chemical properties.
Periods and Groups
• Elements are arranged horizontally in
periods
• the length of each period is determined by
the number of electrons that can occupy
the sublevels being filled in that period.
Periods and Groups
Based on the electron configuration of the
elements, the periodic table can be
subdivided into four sublevels, or blocks.
Sublevel Blocks
• Most reactive, soft, silvery metals.
• Stored under kerosene/oil.
• Are not found free in nature, but as compounds too reactive to exist alone.
• Combine readily with non metals.
• Combine readily with water
•
2 H2O + 2 X → H2 + 2 XOH
• Melting point decreases as you move down the
group.
Alkali Metals Group 1 ( ns1)
Group configuration
Barium
in oil.
• Similar to group 1, but are harder, denser,
stronger, and have higher melting points.
• Less reactive than the alkali metals, but
still too reactive to be found free in nature.
Alkaline Earth metals Group 2 (ns2)
Special Cases: H and He
• By electron configuration H is in
Group 1, but it does not have the
characteristics of an alkali metal.
• By electron configuration He
should be in Group 2, but it does
not have the chemical
characteristics of the group and
acts as a noble gas, so it is put in
Group 18.
• Without looking at the periodic table, give
the group, period, and block in which the
element with the electron configuration
[Xe]6s2 is located.
• Group 2 (ns2)
• Period 6 (6s2)
• Block s (6s2)
Example #1
• Without looking at the periodic table, write
the electron configuration for the Group 1
element in the third period.
• 1s22s22p63s1
Example #2
• Which element is likely to be more active:
• [Xe]6s2 or 1s22s22p63s1 ?
• 1s22s22p63s1 because it is in Group 1 (alkali
metals)
Example #2
• Without looking at the periodic table, give
the group, period, and block in which the
element with the electron configuration
[Kr]5s1 is located.
• Group 1 (ns1)
• Period 5 (5s1)
• Block s (5s1)
Example #3
• Without looking at the periodic table, write
both the group configuration and the
complete electron configuration for the
Group2 element in the 4th period.
• Group ns2
• 1s22s22p63s23p64s2
Example #4
• Sum of d and s electrons give
group number of the element.
Ex. Cr = [Ar] 4s13d5 is in
group 6.
• Metallic properties (shiny,
conduct heat and electricity,
ductile, malleable)
• {ns2(n-1)dx}
Transition metals (d block)
Groups 3-12
• are less reactive than Groups
1 and 2
• Some are so unreactive that
they do not easily form
compounds and can exist in
nature as free elements (also
called native elements).
Transition Metals
Native gold
• An element has the electron configuration
[Kr]5s24d10 Without looking at the periodic
table, identify the period, block, and group
in which this element is located.
• Period 5 (5s2)
• Block d (5s24d10)
• Group 12 (2+10)
Example #4
• The p-block elements together with the s-block
elements are called the main group elements.
• The properties of the elements in the p block
vary greatly (mixture of metals, metalloids, nonmetals, and the noble gases).
• (ns2np1 through ns2np6)
• Group number = ns electrons + np electrons +10
P block elements Group 13-18
• Metalloids: Brittle solids,
semiconductors, exhibit properties of
both nonmetals and metals.
• P-block metals less dense and hard than
d-block metals, but greater than Groups 1
and 2. When pure, are stable in the
presence of air.
• Noble gases=unreactive. Why????
P block elements Group 13-18
• Halogens: most reactive
nonmetals
• React vigorously with
metals, forming salts.
• F2 (green gas), Cl2 (yellow
gas), Br2 (red liquid), I2
(purple solid).
• (ns2np5)
Group 17 – The Halogens
• Stable elements, do not normally undergo
chemical reactions.
• The stability is a result of electrons filling
the highest energy level.
• Helium is 1s2, all the other noble gases are
said to have “stable octets” because the
configuration is ns2 np6 (8 electrons in
outer energy level = octet).
• (ns2np6)
Group 18 - Noble Gases
• Shiny, reactive metals.
• Similar in reactivity to the
Group 2 alkaline earth
metals.
• Electrons fill up f orbitals.
• Atomic # 58-71
Lanthanides (Rare Earths)
• All radioactive metals.
• First 4 naturally occurring.
• The remainder are called
the transuranic elements
(“beyond uranium”), and
are synthesized
• Atomic #90-103
Actinides
• An element has the electron configuration
[Ne]3s23p5 Without looking at the periodic
table, identify the block, and group in
which this element is located.
• Block p (3s23p5)
• Group 17 (2+5+10)
Example #5
• An element has the electron configuration
[Ne]3s23p5 What element is this? Is this a
metal, nonmetal, or metalloid?
• Chlorine
• Group 17 halogen (non-metal), which
means it is very reactive.
Example #6
• An element has the electron configuration
[Xe]6s24f6 What element is this? Is this a
metal, nonmetal, or metalloid?
• This is an f-block element in the
lanthanides (4f). Samarium, Sm.
• Lanthanides are reactive metals.
Example #6
Assignment – Due
tomorrow
• 5.2 Worksheet
Yee-hah!!!