Unit 1 Cells - Inverness Royal Academy
advertisement

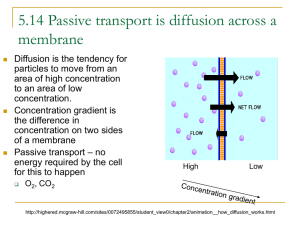
Unit 1 Cells Topic 2 Diffusion and Osmosis Diffusion and Osmosis • Learning objectives: – By the end of the lesson you should be able to: • Describe diffusion as movement of substances like sugar and oxygen in and out of cells. • Explain the importance of diffusion to cells. Diffusion is the movement of a substance from A region of high concentration to a region of where it is at a __________________________________________ Lower concentration until the concentration is equal __________________________________________ Concentration Gradient • The difference between a high and a low Concentration gradient concentration is called a ____________________ Diffuse down a concentration gradient • Substances __ • Diffusion and Cells dioxide, oxygen, dissolved food and waste), • Many substances (carbon ______________________________ important in living processes of a cell, enter and leave diffusion the cell by________________. • These small molecules are able to ______________________ Move through the cell membrane Plant Cell Animal Cell Carbon dioxide Carbon dioxide Waste Oxygen Dissolved food Oxygen Animal Cell Plant Cell Importance of Diffusion • Diffusion is the means by which ________________ Gases are exchanged in living organisms e.g. humans Oxygen Blood with Low oxygen ___________ and High Carbon ___________ _Dioxide concentration Carbon dioxide Blood with _________ High oxygen and ___________ Low carbon ___________ Dioxide Carbon dioxide Oxygen concentration Efficiency of surfaces where diffusion takes place • Where diffusion takes place in organisms the efficiency of the process can be increased by one or more of the following. surface area allowing _____________ Maximum contact • Large ___________ with the diffusing substances. • _______________ often only one cell thick Thin surface Allowing speedy diffusion ___________________________ blood supply to carry away substances • Good _____________________________ that have diffused and so to maintain the concentration gradient. Moist surface • _____________, substances need to be Dissolved in order to diffuse ___________________ Solutions A solution is made from Liquid known as the solvent • ____________________________ e.g. water Dissolved substances known as the solute e.g. salt, glucose • _________________________________ Describing solutions Solutions can be described in a number of ways, these include • Percentage – The solute % plus the solvent % must add up to ______ 100% e.g. a 10% glucose solution is made from 10% glucose and 90% water. – E.g. a 5% salt solution is made up of 5% salt and 95% water Molarity • Solutions can also be described in terms of their molarity • e.g. 1 Molar glucose solution (1M) The higher the number the more solute is in the solution • e.g. a 2 M solution contains more solute than a 1M solution Diffusion and Osmosis • Learning objectives: – By the end of the lesson you should be able to: • Explain the movement of water in plant and animal cells by the process of osmosis. • Describe the effect of osmosis on plant and animal cells. Osmosis • Osmosis is the diffusion of WATER from a HIGH concentration to a LOW concentration across a SELECTIVELY PERMEABLE MEMBRANE Membranes Membranes in cells are Freely permeable to very small molecules • _____________________________________ e.g oxygen Slowly permeable to slightly larger molecules • ______________________________________e.g. sugar Impermeable to very large molecules • ______________________________________ e.g. starch Mechanism of Osmosis • Osmosis is the diffusion of water through a selectively permeable membrane. • Water _______________________________________ Will move from where the water concentration is higher ____________________________________________ To where the water concentration is lower Selectively Permeable Membrane 5% Sucrose Solution 10% Sucrose Solution Sucrose molecule Water molecule Higher Water Concentration Lower water concentration Water will move in both directions but there will be more movement in this direction. Diffusion and Osmosis • Learning objectives: – By the end of the lesson you should be able to: • Describe the effect of placing cells in a HYPERTONIC solution. • Describe the effect of placing cells in an ISOTONIC solution. • Describe the effect of placing cells in a HYPOTONIC solution. Comparing Solutions Terms of their concentration • Solutions can be compared in _____________________ Isotonic same • ___________ solutions have the __________ concentrations of water More water A hypotonic solution contains _____________ • _____________ than the solution which it is compared. hypotonic • E.g. a 10% glucose solution is said to be _________ when compared to a 20% glucose solution as it More water contains ____________ than the 20% solution • ______________ solution contains ______ water A hypertonic less than the solution with which it is compared. • E.g. a 10% glucose solution is said to be ________ hypertonic when compared to a 5% glucose Less water than the 5% solution as it contains _________ solution Distilled water ______________________ is completely pure and so is 100% water, it is hypotonic to all other solutions ____________________________________________ Osmosis and animal cells • When _____________________________________ Cells are placed in solutions of different concentrations then osmosis will take place • E.g. osmosis in red blood cells Appearance of red blood cell Surrounding solution Explanation Isotonic Water enters and leaves the cell at the same rate No net movement of water No change in appearance hypotonic Water flows into the cell Cell swells and bursts This is called haemolysis hypertonic Water flows out of the cell Cell shrinks Osmosis in Plant Cells Appearance of plant cells Surrounding Solution Explanation Isotonic Water enters and leaves the cell at the same rate No net movement of water No change in appearance Hypotonic Water flows into the cell and vacuole swells Cell wall prevents bursting Cell is turgid Hypertonic Water flows out of the cell and vacuole shrinks Membrane peels away from wall. Cell is plasmolysed Osmosis and Tissues Pieces of tissue e.g. potato cylinders can be placed in different solutions Appearance of potato cylinder Surrounding solution Description Isotonic Normal length, diameter _________________ ___________of cylinder And weight Hypotonic Longer, wider and heavier •_______________ than cylinder in isotonic solution •Turgid Shorter, thinner and lighter ___________________ •Than cylinder in isotonic solution •Feels rubbery •________ Flaccid Hypertonic Movement of water by Osmosis • Where two cells are touching and _____________ The solutions inside ________________________________________ have different concentrations water will move from one cell to another ____________ 5% solution 95% water Cell A 6% solution 94% water Cell B Water will move from • ______________________ cell __ A to cell ____ B