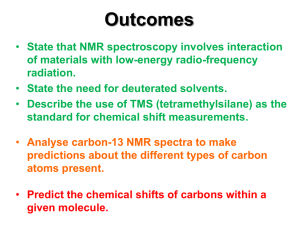
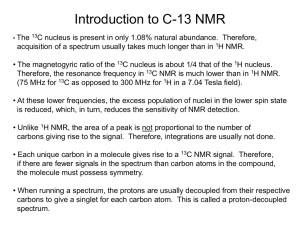
In Figure 1b, the 1H NMR spectra of the macroinitiator and
advertisement
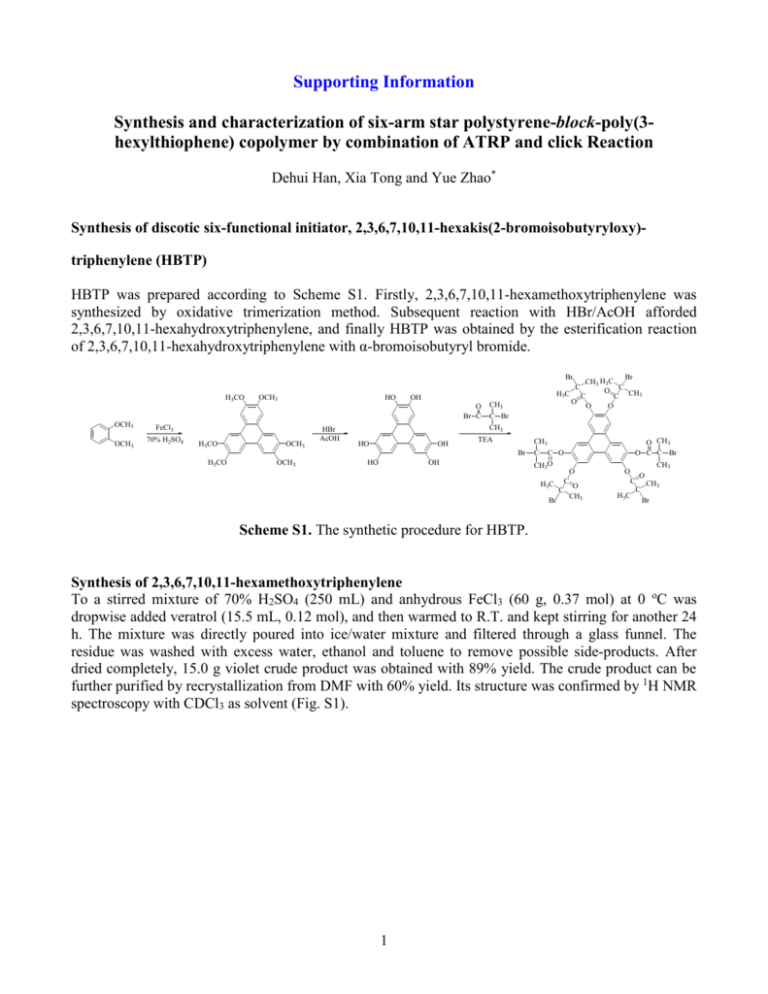
Supporting Information Synthesis and characterization of six-arm star polystyrene-block-poly(3hexylthiophene) copolymer by combination of ATRP and click Reaction Dehui Han, Xia Tong and Yue Zhao* Synthesis of discotic six-functional initiator, 2,3,6,7,10,11-hexakis(2-bromoisobutyryloxy)triphenylene (HBTP) HBTP was prepared according to Scheme S1. Firstly, 2,3,6,7,10,11-hexamethoxytriphenylene was synthesized by oxidative trimerization method. Subsequent reaction with HBr/AcOH afforded 2,3,6,7,10,11-hexahydroxytriphenylene, and finally HBTP was obtained by the esterification reaction of 2,3,6,7,10,11-hexahydroxytriphenylene with α-bromoisobutyryl bromide. Br CH3 H3C C O C CH3 C O O O Br H3CO OCH3 HO H3C OH O Br C OCH3 OCH3 FeCl3 70% H2SO4 H3CO OCH3 HBr AcOH HO OH CH3 C Br CH3 TEA OCH3 HO O CH3 O C C Br CH3 Br H3CO C OH C C O CH3O H 3C Br C C O O CH3 CH3 O C H3C O C CH3 Br Scheme S1. The synthetic procedure for HBTP. Synthesis of 2,3,6,7,10,11-hexamethoxytriphenylene To a stirred mixture of 70% H2SO4 (250 mL) and anhydrous FeCl3 (60 g, 0.37 mol) at 0 oC was dropwise added veratrol (15.5 mL, 0.12 mol), and then warmed to R.T. and kept stirring for another 24 h. The mixture was directly poured into ice/water mixture and filtered through a glass funnel. The residue was washed with excess water, ethanol and toluene to remove possible side-products. After dried completely, 15.0 g violet crude product was obtained with 89% yield. The crude product can be further purified by recrystallization from DMF with 60% yield. Its structure was confirmed by 1H NMR spectroscopy with CDCl3 as solvent (Fig. S1). 1 Figure. S1. 1H NMR spectrum of 2,3,6,7,10,11-hexamethoxytriphenylene. Synthesis of 2,3,6,7,10,11-hexahydroxytriphenylene 2,3,6,7,10,11-hexamethoxytriphenylene (2.02 g, 4.95 mmol) was suspended in a mixture of HBr and AcOH (120 mL, 1:1) and the reaction solution was heated at reflux for 24 h. After stop reaction, the mixture was allowed to cool to R.T. slowly. The precipitate formed was filtered and washed with excess water, then dried to afford a dark purple solid that was further recrystallized from water (1.36 g, 80.5% yield). 1H NMR was used to characterize its structure, as shown in Fig. S2. Figure. S2. 1H NMR spectrum of 2,3,6,7,10,11-hexahydroxytriphenylene (D6-DMSO as solvent). Synthesis of 2,3,6,7,10,11-hexakis(2-bromoisobutyryloxy)triphenylene (HBTP) Into a 50 mL three-neck round flask, 2,3,6,7,10,11-hexamethoxytriphenylene (1.0 g, 2.92 mmol) was added in the presence of argon. Anhydrous DCM (50.0 mL) and freshly distilled triethylamine (1.8 mL, 12.9 mmol) were added by syringe. The suspension was cooled with an ice bath and thoroughly deoxygenated by bubbling with argon for about half an hour. Then, α-bromoisobutyryl bromide (1.38 2 mL, 11.2 mmol) was added dropwise into the mixture with stirring. After reaction for 36 h at room temperature, the salt formed was filtered with a short silicate column. Another 100 mL of DCM was added into the filtrate. The DCM solution was washed with saturated NaCl aqueous solution several times and dried with anhydrous MgSO4. After filtration, DCM was removed completely under reduced pressure and a crude product was obtained that was further recrystallized from CHCl3/EtOH three times. Lastly a 2.2 g of pure product with white colour was obtained with 60% yield. Its structure was confirmed by 1H NMR spectroscopy with CDCl3 as solvent (Fig. S3). Figure. S3. 1H NMR spectrum of HBTP. Figure. S4. 1H NMR of alkynyl-terminated P3HT. 3