Homeostasis of body fluids
advertisement

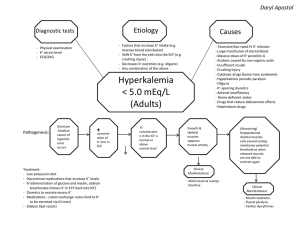
Homeostasis of body fluids G. Ogweno Alle Ding sind Gift, und nichts ohn Gift; allein die Dosis macht, daß ein Ding kein Gift ist "All substances are poisons; there is none which is not a poison. The right dose differentiates a poison and a remedy.” Paracelsus (1493-1541) (Philippus Theophrastus Aureolus Bombastus von Hohenheim ) Fluid compartments compart ment Total Body water ICF %body wt 60% Vol(L) 40% 28 ECF(IS 20(15& F/plasm 5) barrier 42 Cell membra ne 14(10.5 Capillar &3.5) y wall Ionic composition ion units ECF ICF Na+ mEq/L 135-147 10-15 K+ mEq/L 3.5-5.0 120-150 Cl- mEq/L 95-105 20-30 HCO3 mEq/L 22-28 12-16 Ca++ Mmol/L 2.1-2.8 10-7 Pi Mmol/L 1.0-1.4 0.5-0.7 Fluid exchange between ICF and ECF • Free movement of water • Direction by hydrostatic pressure and osmotic pressure balance determined by osmolality • Water channels(aquaporins) • Ionic movement determined by membrane transporters Mechanisms creating ionic gradients • predominantly Na+&Cl- extracellular, K+,Prot&Pi intracellular • Result of active transport e.g Na+/K+ ATPase • Specific channels e.g K+leakage • Presence of large organic osmolytes • Membrane characteristics Membrane transporters Water channels: aquaporins Ion channels: Na+,K+,Ca++,Cl-,Anion,Cat Solute carriers: • Uniport:glucose(Glut2),Fructose(Gluts),Urea(UTA),Fe++(ferroportin) • Symport:Na+-glucose(SGLT2),2Na+-amino acid,Na+-Cl-,Na+K+,Na+-Pi,Na-3HCO3,K+-Cl etc • Antiport: Na+-H+,Cl- -HCO3,3Na+-Ca++ Transport ATPase: • P-Type: Na+,K+ • H+,K+ • H+,Ca++(PMCA) • V-Type:H-ATPase • ABC transporters:cystic fibrosis transmembrane regulator (CFTR),multidrug resistance protein (MRP-1),Organic anion Role of ionic gradients • Optimal enzymatic pH • Membrane potential for excitable tissues • Regulation of cell volume/turgor/shape Effect of exogenous fluid tonicity on ECF • Isotonic NaCl to ECF-increase vol by same amount, ICF unaffected • Hypotonic NaCl to ECF-osmotic equilibration, both ECF&ICF increase, equal but lower osmolality • Hypertonic NaCl-osmotic equilibration, shift from ICF to ECF Regulation of cell volume:isotonic • Water freely permeable, depends on osmotic pressure/osmolality gradient • Function of balance of active ion transporters and GibbsDonnan effect • Can be modified by reduced ATP synthesis or metabolic inhibitors e.g oubain • Steady state determined by impermeant solutes • Permeant solutes only cause transient changes in volume • The greater the permeability of membrane to permeant solutes, the more the rapidity of time-cause of transient changes Response to hypotonic solution Response to hypertonic solution Regulation of cell volume:nonisotonic • ECF normally isotonic • Certain regions non-isotonic e.g renal medulla, brain • Regulation by organic osmolytes e.g sorbitol, myoinositol, methylamines,tarine, glutamate, β-Alanine • Ionic transporters e.g Na+/K+; Na+/H+; • Specific ion channels e.g K+,Cl-, • Cell size monitored by cytoskeleton, macromolecular crowding, ionic strength, stimuli like stretching, second mesengers, neurotransmitters e.g glutamate Measurement of fluid volumes: principles Indicator dilution techniques Qualities of dye: • Easy to detect conc, • equal equilibration rapidly, Complete mixing • Not adsorbed onto cells or constituents • nil disturbance of compartment • Stays in compartment • Can account for elimination e.g by metabolism or excretion • Non toxic Measurement of fluid volumes:agents • Plasma volume:tagged plasma proteins e.g Evans blue(T-1824) or iodine (131I) on albumin • Total blood volume=plasma volX 100/(100-Ht) • Red cell vol=TBV-plasma vol;tagged RBC space e.g by 51 Cr,59Fe, 32 p • ECF: inulin, mannitol, substraction from TBW • Interstitial: indirectly • ISF=ECF-plasma volume • ICF=TBW-ECF • TBW: deuterium(D2O),tritium oxide, aminopyrine Importance of water in life • Cell life-cell turgor and shape • medium for Chemical and metabolic reactions • Transport of nutrients • Body temperature regulation • Elimination of waste • Lubricant finctions