Version 2012 Updated on 030212 Copyright © All rights reserved
Dong-Sun Lee, Prof., Ph.D. Chemistry, Seoul Women’s University
Chapter 4
Aqueous Solutions and
Chemical Equilibria
Classification of electrolytes
Electrolytes form ions when dissolved in water or certain other solvents and
produce solutions that conduct electricity.
Strong electrolytes ionize essentially completely in a solvent, whereas weak
electrolytes ionize only partially.
A salt is produced in the reaction of an acid with a base.
Strong
Weak
1. Inorganic acids such as HNO3,
HClO4, H2SO4*, HCl, HI, HBr,
HClO3, HBrO3
1. Many inorganic acids, including
H2CO3, H3BO3, H3PO4, H2S, H2SO3
HF
2. Alkali and alkaline-earth
hydroxides
2. Most organic acids
3. Most salts ( ex. NaCl, CH3COONa) 3. Ammonia and most organic bases
4. Halides, cyanides, and
thiocyanates of Hg, Zn, and Cd
* H2SO4 is a strong electrolyte, however, HSO4– is a weak electrolyte.
Apparatus for demonstrating conductivity of electrolyte solutions.
Theory of acid and base
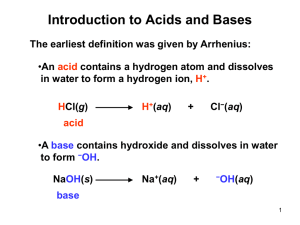
1) Arrhenius’ definition
Acid = a substance that increases the concentration of H3O+ when added to water
HA + H2O = A– + H3O+
Base = a substance that yields OH– ion in aqueous solution
B + H2O = BH+ + OH–
Except for the fact that hydrogen ions neutralize hydroxyl
ions to form water, no complementary relationship between
acids and bases is evident, rather, their oppositeness of
character is emphasized.
Moreover, no account is taken of the behavior of acids and
bases in non-aqueous solvents.
Also, while acidity is associated with so elementary a particle
as the proton (hydrogen ion), basicity is attributed to so
relatively complex an association of atoms as the hydroxyl
ion.
Svante Arrhenius (18591927), Swedish chemist.
Possible structures for the hydronium ion.
a)
The species H9H4+ has been observed in the solid state and may be an important
contributor in aqueous solution.
b)
The species (H2O)21H+ exhibits a dodecahedral caged in the hydrogen bonded
cage with 10 non-hydrogen-bonded protons protruding from its surface.
2) Proton concept ( Brönsted-Lowry definition)
Acid = proton donor
A = B + H3O+
Base = proton acceptor
B + H3O+ = BH+ + OH–
It is apparent that not only molecules but also cations and anions may function as
acids and bases.
Any actual manifestation of acid-base behavior must involve interaction between two
sets of conjugate acid-base pairs ;
A1 + B2 = B1 + A2
Protolysis or protolytic reaction
conjugated acid-base pairs
Ex.
HCl + H2O =
Cl– + H3O+
NH3 + H2O =
NH4+ + OH–
water = amphiprotic substance
CH3COOH + CH3NH2 = CH3COO– + CH3NH3+
3)
Electron pair concept ( Lewis definitions )
Acid = electron pair acceptor
Base = electron pair donor
Ex. 1) H3O + + :OH– = H2O + H:OH
2) BCl3 + :NH3 = Cl3B:NH3
3) in non-aqueous solvent
SbCl5 + :Cl– = [SbCl6]–
Three general types of solvents :
1) Protic solvent : amphiprotic solvents
-
possess both acidic and basic properties
2SH = SH4+ + S–
undergoes self-ionization (=autoprotolysis)
2H2O = H 3O+ + OH–
ex. Water, lower alcohols, acetic acid, ammonia
ethylenediamine
2) Aprotic solvents
-
have no appreciable acidic or basic character
-
do not undergo autoprotolysis
-
ex. Benzene, carbon tetrachloride, pentane
3) Basic solvents
-
have basic properties but essentially no acidic
tendencies
-
do not undergo autoprotolysis
ex. Ketones, ethers, esters, pyridine and amines
2C2H5OH = C2H5OH2+ + C2H5O–
2HOAc = H2OAc+ + OAc–
2NH3 = NH4+ + NH2–
Strengths of Acids and Bases
In a differentiating solvent, various acids dissociate to different degrees and
have different strengths.
In a leveling solvent, several acids are completely dissociated and show the
same strength.
The leveling effect of water
For an aqueous solution, the strongest possible acid is H3O+, and the strongest
possible base is OH–. The reason for this is that each one of the acids undergoes
practically complete protolysis in water.
HClO4
+
H2O
H2SO4
HCl
HNO3
NaH
+
HCl + H2O = Cl– + H3O+
acid
base
acid
H3O+
H2O
NaOC2H5 + H2O = C2H5OH + OH–
NaNH2
NaOC2H5
base
OH –
Acid and base strengths that are not distinguished in aqueous solution may be
distinguishable in non-aqueous solvents.
Ex. Perchloric acid is a stronger acid than hydrochloric acid in acetic acid solvent,
neither acid is completely dissociated.
HClO4 + CH3COOH = ClO4–
strong acid
base
weak base
HCl + CH3COOH = Cl–
+ CH3COOH2+
K = 1.3×10–5
weak acid
+ CH3COOH2+
K = 5.8×10–8
Differentiate acidity or basicity of different acids or bases
differentiating solvent for acids …… acetic acid, isobutyl ketone
differentiating solvent for bases
…… ammonia, pyridine
Strong acids and bases
When strong acid such as HCl is added to water, the following reactions occur :
HCl + H2O = Cl– + H3O+
2H2O
= H3O+ + OH–
The charge balance :
[H3O+] = [Cl–] + [OH–]
In most instances ( [HCl] > 4.5×10–7 M) the [OH–] would be negligible compared to the
[Cl–], therefore the charge balance equation simplifies to
[H3O+] = [Cl–] = [HCl]
0.1 M HCl
[H3O+] = 0.1 M
Key points
Strong acid
or strong base
pH = – log (0.1) = 1.00
Completely
dissociate
[CAcid] = [H3O+ ]
[CBase] = [OH–]
HCl fountain
Strengths of acids and bases
Acids and bases are commonly classified as strong or weak, depending on whether they
react “completely” or only “partly” to produce H+ or OH–.
Strong acids and bases
A strong acid or base is completely dissociated in aqueous solution.
The equilibrium constant of the following reactions are very large.
HCl (aq) = H+ + Cl–
KOH (aq) = K+ + OH–
Common strong acids and bases
HCl
Hydrochloric acid
LiOH
Lithium hydroxide
HBr
Hydrogen bromide
NaOH
Sodium hydroxide
HI
Hydrogen iodide
KOH
Potassium hydroxide
H2SO4
Sulfuric acid
RbOH
Rubidium hydroxide
HNO3
Nitric acid
CsOH
Cesium hydroxide
HClO4
Perchloric acid
R4NOH
Quaternary ammonium hydroxide
Ex. 1) 0.10M HBr pH=?
pH = – log (0.1) = 1.00
[HBr] = [H+] = 0.1
2) Ionic strength of 0.1 M HBr = 0.1 M
pH = – log [H+] f = – log (0.10)(0.83) = 1.08
3) 0.10M NaOH pH = ?
[OH–] = 0.10 M
pOH =1.00
pH = pKw – pOH = 14.00 – 1.00 = 13.00
4) 1.0 ×10–8 M NaOH
[OH–] = 1.0 ×10–8 M
pH = ?
pOH =8.00
pH = pKw – pOH = 14.00 – 8.00 = 6.00
This is definitely wrong ! since NaOH is basic.
How overcome this dilemma ?
The pH of very dilute acid or base
Ex.
1.0 ×10–8 M NaOH
pH = ?
If [Cacid or base] <10–6M,
the contribution of water ionization
must be taken into account.
1) NaOH Na+ + OH–
H2O = H+ + OH–
2) [Na+] + [H+] = [OH–]
3) [Na+] = 1.0 ×10–8 M
4) Kw = [H+][OH–] = 1.0 ×10–14
5) Three equations, three unknowns.
6) Kw = [H+][OH–] = [H+] ([Na+] + [H+])
= x(1.0 ×10–8 + x) = 1.0 ×10–14
x2 +(1.0 ×10–8) x– 1.0 ×10–14 = 0
x = 9.6×10–8 or – 1.1 ×10–7
[H+] = x = 9.6×10–8
pH = 7.02
Eminently
reasonable
The pH of very dilute acid or base
Ex.
1.0 ×10–7 M HCl
pH = ?
1) HCl H+ + Cl–
HCl must be considered the
minor supplier of H+, its
contribution is neglected.
2) [H+] = [Cl–] + [OH–]
3) [Cl–] = 1.0 ×10–7 M
–
4) Kw = [H ][OH ] = 1.0
Ex. 3. 1.0 ×10–10 M HCl
pH = ?
H2O = H+ + OH–
+
Only the ionization of water is
considered.
×10–14
5) Three equations, three unknowns.
6) Kw = [H+][OH–] = [H+] ([H+] – [Cl–])
H2O = H+ + OH–
Kw = [H+][OH–]
= x( x –1.0 ×10–7) = 1.0 ×10–14
x2 = 1.0 ×10–14
x2 –(1.0 ×10–7) x– 1.0 ×10–14 = 0
[H+] = x = 1.0×10–7
[H+] = x = 1.62×10–7
pH = 6.79
pH = 7.00
Calculation of pH : strong acids and bases
Key points
Case Major supplier of H+ Necessary condition
1.
Strong acid or base
Cacid or base >> 10–6 M
2.
Both
10–6 < Cacid or base < 10–8 M
3.
Water
Cacid or base << 10–8 M
Calculated pH as a function of the
concentration of a strong acid or
strong base dissolved in water.
General concepts of chemical equilibrium
aA + bB
=
cC + dD
Products
Reactants
Equilibrium constant :
[C]c[D]d
K=
Standard state :
[1M] or [1atm]
[A]a[B]b
pure solids, liquids, solvent =1
Cato Guldberg (1836-1902) and Peter Waage (1833-1900) were Norwegian
chemists whose primary interests were in the field of thermodynamics. In 1864,
these wokers were the first to propose the law of mass action.
Criteria of equilibrium
1) Macroscopic level : no changes in the bulk properties with
the passage of time ; static.
2) Microscopic (molecular) level : equal the rates of the
forward and reverse reactions ; dynamic.
3) Minimum energy change : the composition corresponds to
a minimum in the energy of the reactants and products.
4) Mathematical expression : equilibrium constant, K.
Law of chemical equilibrium : Law of mass action
A and B disappearing
Equilibrium
concentration
C and D appearing
Initial
state
0
Time
Change
Equilibrium
Progress of chemical reaction. A+B = C+D
Le Châtelier’s principle and chemical equilibria
When a system in dynamic equilibrium subjected to a disturbance that upsets the
equilibrium, the system changes in a way to reduce the disturbance and, if
possible, return to equilibrium.
1) Changes in the concentration of a reactant or product
The position of equilibrium shifts in a direction from a substance
that has been added.
The position of equilibrium shifts in the direction of a substance
that has been removed.
RP
reaction quotient :
Q = [P] / [R] K
if Q = K equilibrium
Q < K forward reaction, spontaneous
Q > K reverse reaction, spontaneous
F
A+B = C+D
R
+
excess
Ex.
BrO3– + 2Cr3+ + 4H2O
= Br– + Cr2O72– + 8H+
K = 1×1011 (25oC)
Rate of reaction
A
Addition of
excess A
F
FR
R
FR
New
equilibrium
Initial
equilibrium
[Cr2O72–] = 0.10M 0.20 M
Q = [(1.0)(0.20)(5.0)8]/[(0.043)(0.003)2]
= 2 ×1011 > K
Time
2) Temperature effects on equilibrium constants
K=e
–Go/RT
=e
–(Ho –TSo)/RT
=e
(–Ho/RT+So/R)
=e
–Ho/RT•
This term increases with
increasing T if Ho>0
e
So/R
Independent term
Endothermic reaction : T K
Exothermic reaction :
T K
Ex. N2 (g) + O2 (g) = 2NO(g) Ho =+181kJ
N2 (g) +3H2 (g) = 3NH3(g) Ho = –92.2kJ
if T Forward
if T Reverse
Free energy and equilibrium
Go = Ho –TSo
Go = –RTlnK
lnK = –Go / RT
K = e –Go/RT
Ex.
HCl = H+ + Cl–
Go = Ho –TSo
= (–75.15×103J) – (273.15+25K)(–131.5J/K)
= – 35.94 kJ/mol
K = e –(–35.94×1000J/mol)/[8.31441 J/(Kmol)](298.15K)
= 1.98 ×106
3) Pressure effects on equilibrium constants
In the gaseous phase, pressure can have large influence
on the position of equilibrium, therefore it changes K.
Decreasing the volume of a mixture of gases that are in
chemical equilibrium shift the position of equilibrium in
the direction of the fewest number of molecules of gas.
Ex. N2(g) + 3H2 (g) = 2NH3(g)
4 moles
if P forward
2 moles
Changing the external pressure on a chemical system
containing only liquids and solids has virtually no effect
on the position of equilibrium.
Categories of most reactions used in chemical analysis
1) Dissociation
HA = H+ + A–
2+
K = [H+ ][A–] / [HA]
–
2+
– 2
MA2 (s) = M + 2A
Ksp = [M ][A ] / [MA2]
H2O = H3O+ + OH –
Kw = [H3O+][OH–]
2) Formation
M2+ + A2– = MA
K = [MA] / [M2+ ][A2–]
3) Redox
Ox1 + Red2 = Red1 + Ox2
K = [Red1][ Ox2] / [Ox1][ Red2]
4) Distribution equilibria
X(aq) = X(org)
Kd = [X](aq) / [X](org)
Ionization and pH of water / Ion product constant for water
H2O = H+ + OH–
Kw = AH+ AOH– = [H+ ]fH+ [OH–]fOH– = 1.008 ×10–14 (25oC)
[H+] = [OH–] = 1.0 ×10–7
pH = – log AH+ = – log[H+ ]fH+ = 7.00
pKw = pH + + pOH–
Ex. [H+] in 0.10M KCl at 25 oC
ionic strength = 0.10M
Kw = AH+ AOH– = [H+ ]fH+ [OH–]fOH– = 1.008 ×10–14
= xfH+ xfOH– = x (0.83) x (0.76) = 1.008 ×10–14
x = 1.26 ×10–7
AH+ = [H+ ]fH+ = (1.26 ×10–7 )(0.83) = 1.05 ×10–7
pH = – log AH+ = – log[H+ ]fH+ = 6.98
matrix effect
Temperature dependence of pH
Body temperature = 37 oC
Blood pH = 7.35 ~ 7.45
[HCl] in gastric juice = 0.1 ~ 0.02M
if [H+] = 0.02M
pH = 1.7
pOH = 13.6 –1.7 = 11.9 at 37 oC
pH of various substances
Solubility product constant , Ksp
The solubility product is the
equilibrium constant for the
reaction in which a solid salt
dissolves, to give its constituent
ions in solution.
The concentration of the solid is
omitted from the equilibrium
constant because the solid is in
its standard state.
Ex. AB(s) = A+(aq) + B–(aq)
+
–
Ksp = K[AB] = [A ][B ]
KspIP supersaturation(ppt)
Example
Dissociation of mercurous chloride
Hg2Cl2 = 2Hg+ + 2Cl–
( ×)
Hg2Cl2 = Hg22+ + 2Cl–
( O)
Initial
0
0
Final
x
2x
Ksp = [Hg22+][Cl–]2 = (x)(2x)2
3
= 4x =1.2×10
-18
x =[(1.2×10-18)/4]1/3
-7
= 6.7×10 M
Common ion effect
Ex. Concentration of mercurous ion in a
soln containing 0.030M NaCl saturated
with mercurous chloride
A salt will be less soluble if
one of its constituent ions is
already present in the solution.
2+
– 2
Cf. Ksp = [Hg2 ][Cl ]
= (x)(2x)2
3
= 4x =1.2×10
-18
x = 6.7×10-7 M
Hg2Cl2 = Hg22+ + 2Cl–
Initial
0
Final
x
0
2x +0.03
Ksp = [Hg22+][Cl–]2
= (x)(2x+0.03)2 =1.2×10-18
2x<<0.03 2x+0.03 0.03
Ksp (x)(0.03)2 =1.2×10-18
x = 1.3×10-15 M
Separation by precipitation: selective or fractional precipitation
Ag+
CrO42– ,
Cl -
2 Ag+ + CrO4 2 – = Ag2CrO4
Ag+ + Cl – = AgCl
Ksp = (2x) 2(x) = 1.1×10-12M
Ksp = (x)(x) = 1.0×10-10M
x = 6.5×10-5M
x = 1.0×10-5M
[Ag+] = 2x = 1.3 ×10-4M
[Ag+] = x = 1.0 ×10-5M
Effects of complex formation on solubility
When a complex is formed by the metal ion of an insoluble
salt, the solubility of the salt is increased.
Ex. 1)
AgCl(solid) = Ag+ (aq) + Cl– (aq)
Ag+ (aq) + 2NH3 (aq) = Ag(NH3)+ (aq)
soluble complex
2) Al(OH)3 (solid) = Al+3 (aq) + 3OH– (aq)
Al+3 (aq) + 6F– (aq) = AlF6–3 (aq)
soluble complex
Weak acids and bases : small Ka or Kb
acid dissociation constant : Ka
HA + H2O = A– + H3O+
Ka = AA– AH3O+ /AHA
[A–] [H3O+] / [HA]
base dissociation constant : Kb
B + H2O = BH+ + OH–
Kb = ABH+ AOH– /AB
[BH+] [OH–] / [B]
Common types of weak acids and bases
All carboxylic acids are weak acids, and all carboxylate anions are weak bases.
RCOOH =
RCOO– + H+
Amines are weak bases, and ammonium ions are weak acids.
RNH2 = RNH3+ + OH–
R2NH = R2NH2+ + OH–
R3N = R3NH+ + OH–
ex. CH3COOH = CH3COO– + H+
Ka = 1.75×10–5
CH3NH2 = CH3NH2+ + OH–
Kb = 4.4 ×10–4
pKa
pKa = –log ( AA– AH3O+ /AHA )
–log ( [A–] [H3O+] / [HA] )
pKb –log ( [BH+] [OH–] / [B] )
Relation between Ka and Kb
HA = A– + H+
Ka = [A–] [H3O+] / [HA]
A– + H2O =
Kb = [HA] [OH–] / [A–]
HA + OH–
H2O = H+ + OH–
Kw
Ka Kb = Kw
Ka1 Kb2 = Kw
Ka2 Kb1 = Kw
Ka1 Kb3 = Kw
Ka2 Kb2 = Kw
Ka3 Kb1 = Kw
Ex. Finding Kb for the conjugate base
HAC
Ka = 1.75×10–5
AC–
Kb = ?
Kb = Kw/ Ka = 1.0×10–14 / 1.75×10–5 = 5.7×10–10
Ex. Finding Ka for the conjugate acid
methylamine
Kb = 4.4×10–4
Ka = Kw/ Kb = 2.3×10–11
pKa = 10.64
methylammonium ion
pKa = ?
Ex.
pH of weak acid solution
1) Calculate the pH and pOH of 1.00 ×10–3 M aqueous solution of CH3COOH
having a Ka = 1.75×10–5
Solution :
HOAC = H+ + OAC–
initial 1.00×10–3
final
0
0
1.00×10–3–x x
x
Ka = [OAC–][H+] / [HOAC] = x2 / (1.00×10–3 – x)
1.00×10–3>> x ,
1.00×10–3 – x 1.00×10–3
Ka x2 / 1.00×10–3 =1.75×10–5
x [H+] 1.32×10–4
pH = – log 1.32×10–4 = 4 – log 1.32 = 4 – 0.12 = 3.88
pOH = 14.00 – 3.88 = 10.12
Ex. Calculation of the pH of weak acids.
COOH
Benzoic acid
Ka = 6.28 ×10–5
1) 0.0500 M benzoic acid pH=?
Ka = [A–][H+] / [HA] = (x)(x) / (F –x)
x2 + Kax – KaF = 0
x = ( – Ka+ Ka2 +4KaF) / 2
x = [H+] = 1.77 × 10–3
pH = – log (1.77 × 10–3 ) = 2.75
KaF >>Kw , F >>x ,
COOH
OH
Ka1 =
x = [H+] = KaF = 1.77×10–3
2) 0.0500 M
Salicylic acid
1.07×10–3
F –x F .
Answer
salicylic acid
pH= 2.75
pH=?
x = ( – Ka+ Ka2 +4KaF) / 2
x = [H+] = 7.31× 10–3
Ka2 = 1.82×10–14
pH = 2.14
HO
COOH
p-Hydroxybenzoic acid
3) 0.0500 M
Answer
p-hydroxybenzoic acid
x = ( – Ka+ Ka2 +4KaF) / 2
Ka1 = 2.63×10–5
x = [H+] = 1.15× 10–3
Ka2 = 8×10–10
pH = 2.94
pH=?
Ex. pH of weak acids
+
H3
O
Cl –
N
CH3
H3C
HN
NH
O
CH3
Trimethyl ammonium chloride
Salts of this type are completely
dissociated
(CH3)3NH3+Cl– (CH3)3NH3+ + Cl–
0.1 M
0.1 M
(CH3)3NH3+ = H+ + (CH3)3NH2
0.1M – x
O
x
x
Ka = (x)(x) / (0.1 –x) x2/ 0.1 =1.58×10–10
x = [H+] = 3.97×10–6
pH= 5.40
Barbituric acid
HA
=
H+ + A–
0.01M – x x
x
Ka = (x)(x) / (0.01 –x)
x2 / 0.01 =9.8×10–5
x = [H+] = 9.90×10–4
pH= 3.00
Manipulation of equilibrium constants
+
–
+
–
HA = H + A
K1 = [H ][A ] / [HA]
MA2 (s) = M2+ + 2A–
K2 = [M2+ ][A–]2 / [MA2]]
HA + MA2 (s) = H+ + 3A– + M2+
K3 = ?
K3 = [H+][A–][M2+][A–]2 / [HA][MA2]
= K1 K2
Polyprotic acids and bases
Polprotic acids and bases are compounds that can donate or accept more than one proton.
Monoprotic :
HA = A– + H+
B
Diprotic :
Triprotic :
= BH+ + OH–
H2A = HA– + H+
Ka1
HA– = A–2 + H+
Ka2
H3A = H2A– + H+
Ka1
H2A– = HA–2 + H+
Ka2
HA– = A–3 + H+
Ka3
(COOH)2 , H2CO3
H3PO4
Ex.
pH of weak base solution
1) Calculate the pH of 0.05 M aqueous solution of CH3COONa
Assume Ka for acetic acid is equal to 1.75×10–5
Solution :
NaOAC Na+ + OAC–
completely dissociate
OAC– + H2O = HOAC + OH–
initial
0.05
0
0
final
0.05–x
x
x
Kb = Kw / Ka = 1.00×10–14 / 1.75×10–5 = 5.71×10–10
= [OH–] [HOAC] / [OAC–] = x2 / (0.05 – x)
0.05>> x ,
0.05 – x 0.05
x [OH–] = KbF = 5.71×10–10 × 0.05 = 5.34 ×10–6 M
pOH = – log 5.34 ×10–6 = 5.27
pH = 14.00 – 5.27 = 8.73
Ex. Calculation of the pH of weak bases.
COO – Na+
Sodium benzoate
Ka = 6.28 ×10–5
COO –
1) 0.0500 M sodium benzoate pH=?
Kb = Kw / Ka = 1.00×10–14 / 6.28×10–5 = 1.59×10–10
x = [OH–] = KbF = 2.82×10–6
pH = 14.00 – 5.55 = 8.45
2) 0.0500 M
salicylic acid
pH=?
Kb = Kw / Ka = 1.00×10–14 / 1.07×10–3 = 9.35×10–12
OH
Salicylic acid
x = [OH–] = KbF = 6.84×10–7
Ka1 = 1.07×10–3
pH = 14.00 – 6.17 = 7.83
Ka2 =
pOH= 5.55
pOH= 6.17
1.82×10–14
3) 0.0500 M
HO
COO –
p-hydroxybenzoic acid
Kb = Kw / Ka = 1.00×10–14 / 2.63×10–5 = 3.80×10–10
p-Hydroxybenzoic acid
x = [OH–] = KbF = 4.36×10–6
Ka1 = 2.63×10–5
pH = 14.00 – 5.36 = 8.64
Ka2 = 8×10–10
pH=?
pOH= 5.36
Ex. Calculation of pH of weak base ( tropane alkaloid)
COOCH3
N—CH3
Cocaine
OCOC6H5
COOCH3
HN+—CH3
=
Kb = 2.6 × 10–6
B + H2O = BH+ + OH–
initial
F
0
0
final
F–x
x
x
H2O = H+ + OH–
Kb = [BH+] [OH–] / [B] = (x)(x) / (F –x) = 2.6 × 10–6
If assume F >>x ,
F –x F .
x2 / (F –x) x2 / F = Kb
[H+]= Kbw / [OH–]
x = [OH–] = KbF
OCOC6H5
Buffer solution
A buffered solution is one that resists change of pH on adding acids or bases, or
on diluting it with solvent.
The buffer consists of mixture of an acid and its conjugated base.
Dissociation equilibria :
HA = A– + H+
Dissociation equilibria constants :
Ka = [A–][H+] / [HA]
[H+] = Ka [HA] / [A–]
– log [H+] = – log Ka [HA] / [A–]
= – log Ka – log [HA] / [A–]
= – log Ka + log [A–]/[HA]
pH = pKa+ log [A–] / [HA]
[A–]/[HA]
pH
100:1 or 1:100
pKa 2
10:1 or 1:10
pKa 1
1:1
pKa 0
Henderson-Hasselbalch
equation
Ex. Tris buffer
NH2
N+H3
C
HOH2C
HOH2C
=
CH2OH
HOH2C
HOH2C
C
+
H+
CH2OH
Tris (hydroxymethyl)aminomethane
Tris hydrochloride(FW=157.597) :
BH+Cl–
BH+ + Cl–
BH+ = B + H+
Find the pH of a solution prepared by dissolving 12.43g of tris plus 4.67g of tris
hydrochloride in 1.00 L of water. pKa = 8.075
Tris = B :
121.136 g/L = 1.00M
Tris hydrochloride = BH+ : 157.597 g/L = 1.00 M
12.43 g/L = x M
x = 0.1026 M
4.67 g/L = y M
y = 0.0296 M
pH = pKa+ log [B] / [BH+] = 8.075 + log (0.1026 / 0.0296)
= 8.61
Effect of dilution of a buffer solution
Ex. 1) Calculate the pH of a buffer prepared by mixing 0.250 mol of acetic acid with
0.100 mol of sodium acetate and diluting to 1.00 L.
pKa = – log 1.75×10–5 = 4.75
2) Calculate the pH when 10.0 ml of this buffer is diluted to 250 mL with water.
HOAC = H+ + OAC–
Ka = [OAC–][H+] / [HOAC]
[H+] = Ka [HOAC] / [OAC–]
pH = pKa+ log [OAC–] / [HOAC] = 4.75 + log ( 0.100M)/(0.250M) = 4.36
HOAC 0.250 M×10.0 ml = x M×250 ml
NaOAC 0.100 M ×10.0 ml = x M×250 ml
x = 0.0100 M
x = 0.00400 M
pH = pKa+ log [OAC–] / [HOAC] = 4.75 + log ( 0.00400M)/(0.0100M) = 4.36
Effect of addition of acids or bases to buffer.
HCl + NaA HA + NaCl
NaOH + HA NaA + H2O
Ex. A buffer consisting of 0.20M HA and 0.10M NaA
pH = pKa+ log [NaA] / [HA] = pKa+ log (0.10/0.20) = pKa+ 0.30
If sufficient strong base is added to react with half of the HA present,
the new concentration of HA will be 0.10M.
The reaction of the strong base with HA will produces some NaA,
making its new concentration 0.20M.
NaOH + HA NaA + H2O
pH = pKa+ log [NaA] / [HA] = pKa+ log (0.20/0.10) = pKa– 0.30
pH = pKa+ 0.30 pKa– ( pKa– 0.30 ) = 0.60
Not very large
Buffer capacity
The relative ability of a buffer solution to resist pH change upon addition of an
acid or a base.
= dCb / dpH = – dCa / dpH
A buffer is most effective in resisting changes in pH when pH= pKa
(i.e., when [HA] = [A–]).
Choose a buffer for an experiment whose pKa is as close as possible to the
desired pH.
The useful pH range of a buffer is usually considered to be pKa 1 pH unit.
(a) Cb versus pH for a solution containing 0.100F HF with pKa =5.00.
(b) Buffer capacity versus pH for the same system reaches a maximum
when pH = pKa. The lower curve is the derivative of the upper curve.
Buffer pH depends on ionic strength and temperature
Changing ionic strength changes buffer pH.
– log f = kZ2 / (1+) – 0.1Z2
pH = pKa+ log [A–]f /[HA]f
Changing temperature changes buffer pH
dpKa / dT = (pKa– 0.9 )/T
Effect of pressure on buffers
High pressure increases the ionization of weak electrolytes, by enhancing the
solvation of the ions, but the effect is not very great.
The composition of buffer solutions as a function of pH: value
CT = CHOAC + CNaOAC
0 : fraction of the total concentration of acid that is undissociated
0 = [HOAC] / CT
1 : the fraction dissociated
1 = [OAC–] / CT
Ka = [OAC–][H3O+] / [HOAC]
[OAC–] = Ka [HOAC] /[H3O+]
CT = CHOAC + CNaOAC = [HOAC] + [OAC–]
= [HOAC] + Ka [HOAC] /[H3O+] = [HOAC] {([H3O+]+ Ka) / [H3O+] }
0 = [HOAC] / CT = [H3O+] / ([H3O+]+ Ka)
1 = [OAC–] / CT = Ka / ([H3O+]+ Ka)
Variation in of HOAC with pH
Buffer capacity as a function of
the logarithm of the ratio
CNaA/CHA. The maximum buffer
capacity occurs when the
concentration of acid and
conjugated base are equal; that is,
when 0 = 1 = 0.5.
Fraction of dissociation : value
The fraction that is in the dissociated form of
5
B
e
n
z
o
i
c
a
c
i
dK
=
6
.
2
6
x
1
0
a
weak acid.
3
S
a
l
i
c
y
l
i
c
a
c
i
d
K
=
1
.
0
7
x
1
0
a
5
p
H
y
d
r
o
x
y
b
e
n
z
o
i
c
a
c
i
d
K
=
2
.
6
3
x
1
0
a
= H+ + A–
HA
1
.
0
F–x
x
= [A–] / ([HA] + [A–])
=
x / {(F–x)+ x}
.
8
x
.
6
x/F
Fractionofdisociaton(
=
Ex. 0.0500 M benzoic acid
Ka =
[A–][H+]
/ [HA] = (x)(x) / (F –x)
x = [H+] = [A–] = 1.77 × 10–3
= x / F = 1.77 ×
= 0.0354
= 3.54 %
10–3
.
4
.
2
0
.
0
0
1
2
3
4
5
/ 0.05
l
o
g
a
r
i
t
h
m
i
c
f
o
r
m
a
lc
o
n
c
e
n
t
r
a
t
i
o
n
o
f
w
e
a
k
a
c
i
d
The fraction of dissociation of weak acids
increases as the acid is diluted.
The fraction of dissociation of weak electrolyte increases as the electrolyte is
diluted. Stronger acid is more dissociated than the weaker acid at all concentrations.
Fraction of association :
The fraction that is in the dissociated form of weak base.
B + H2O =
BH+ + OH–
initial
F
0
0
final
F–x
x
x
H2O = H+ + OH–
= [BH+] / {[B ] + [BH+] }
=
x / {(F–x)+ x}
=
x/F
Ex. 0.0372 M cocaine solution
Kb = 2.6× 10–6
x = [BH+] = [OH–] = KbF = 3.10 × 10–4
= x / F = 3.10 × 10–4 /0.0372
= 0.0083
= 0.83 %
Summary
Strong or weak electrolyte
chemical equilibrium
Acid-base : Brönsted-Lowry definition,
Le Châtelier’s principle
Lewis definitions
Ionization and pH of water
amphiprotic solvents
Solubility product constant , Ksp
Aprotic solvents
Common ion effect
autoprotolysis
selective precipitation
Strong acids and bases
pKa , Ka , Kb, Kw
pH of very dilute acid or base
pH of weak acid solution
differentiating solvent
buffer solution
leveling solvent
Relative concentration,
leveling effect
non-aqueous solvents