CP Chemistry
advertisement
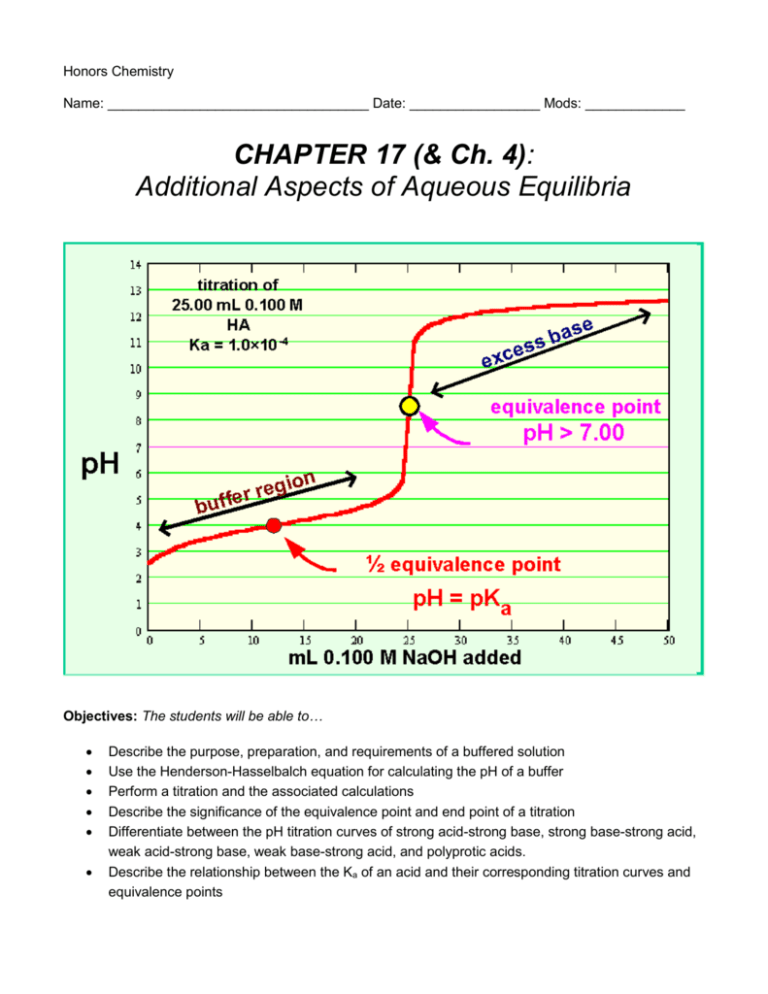
Honors Chemistry Name: __________________________________ Date: _________________ Mods: _____________ CHAPTER 17 (& Ch. 4): Additional Aspects of Aqueous Equilibria Objectives: The students will be able to… Describe the purpose, preparation, and requirements of a buffered solution Use the Henderson-Hasselbalch equation for calculating the pH of a buffer Perform a titration and the associated calculations Describe the significance of the equivalence point and end point of a titration Differentiate between the pH titration curves of strong acid-strong base, strong base-strong acid, weak acid-strong base, weak base-strong acid, and polyprotic acids. Describe the relationship between the Ka of an acid and their corresponding titration curves and equivalence points Chapter 17 (& Ch. 4) Reading Outline: 17.1 – The Common-Ion Effect (pg. 722-724) 1. What is the common-ion effect? 17.2 – Buffered Solutions (pgs. 725-731) 1. Solutions which contain a ______________ conjugate acid-base pair can resist drastic changes in _________ upon the addition of small amounts of ______________ acid or base. These solutions are called ____________________ ___________________ (or _________________). 2. Why does a buffer resist changes in pH? 3. How is a buffer prepared? Give an example of how a buffered solution of HC2H3O2 - C2H3O2would be prepared. 4. Copy equation 17-3 below which details the acid-dissociation equilibrium between a weak acid (HX) and its conjugate base (X-). 5. Copy equations 17-4 (acid-dissociation expression) and 17-5 (which shows that [H+], and thus pH, depends on the concentrations of the conjugate acid-base pair). 6. Copy Figure 17.2 (pg. 726) which explains how a buffered solution is able to neutralize both strong acids (H+) and strong bases (OH-). 7. Copy equation 17.9, known as the Henderson-Hasselbalch equation for calculating the pH of a buffer. 8. For a buffer, when [conjugate base] = [weak acid], what else is also equal? 9. Define buffer capacity: 10. Which has a greater buffering capacity, higher or lower concentrations of the weak acid and its conjugate base? Explain why. 11. Define pH range: 12. How does the pKa of the weak acid in a buffer relate to the pH range? 4.6 – Solution Stoichiometry (pgs.151-156) 1. A titration is an experimental procedure. Use the glossary at the back of the book to define titration. 2. What is a standard solution? 3. Titrations can be conducted using what three types of reactions? 4. What is the equivalence point of a titration? * You will practice examples of titration calculations, which utilize stoichiometry, in class 17.3 – Acid-Base Titrations (pgs. 732-743) 1. While acid-base indicators can be used to signal the equivalence point in a titration, a pH meter can also be used to monitor the progress of the titration reaction and produce a pH titration curve. When graphing a titration curve, pH is a function of what? 2. What are three kinds of titration curves that we will investigate? 3. A titration curve can be divided into 4 regions. Identify and briefly describe each of these regions. (1) (2) (3) (4) 4. Draw the basic pH curve for adding a strong base to a strong acid (Figure 17.6) and label the equivalence point. 5. Draw the basic pH curve for adding a strong acid to a strong base (Figure 17.8) and label the equivalence point. 6. Draw the basic pH curve for adding a strong base to a weak acid (Figure 17.9) and label the equivalence point. 7. The pH titration curves for weak acid- strong base titrations differ from those of strong acid-strong base titrations in three noteworthy ways: (1) (2) (3) 8. The figure to the right (similar to Figure 17.11) represents the effect of Ka on titration curves. One thing this chart illustrates is that, as expected, the initial pH of the weak acid solution is always _________________ than that of the strong acid at the same concentration. Notice also that the pH change near the equivalence point becomes ___________ marked as the acid becomes weaker (that is Ka becomes ____________________). Finally, the pH at the equivalence point steadily ____________________ as Ka decreases. Because the pH change near the equivalence point becomes _________________ as Ka decreases, the choice of indicator for a ____________ acid-strong base titration is more critical than it is for a _____________ acid-strong base titration. 9. Draw the basic pH curve for adding a strong acid to a weak base (Figure 17.12 – blue curve) and label the equivalence point. 10. Titrations of Polyprotic Acids: When weak acids contain more than one ionizable H atom, the reaction with OH- occurs in a series of steps. When each neutralization step is sufficiently separated (sufficient difference in Ka), the substance exhibits a titration curve with ___________________ equivalence points. 11. Draw the basic pH curve for the titration of a polyprotic acid (Figure 17.13) and label each point on the curve.