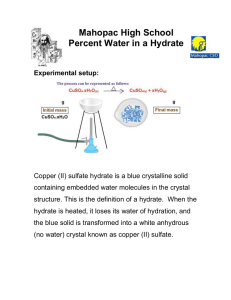
Unit Review: The Mole Determine the empirical formula of a
advertisement

Unit Review: The Mole 1. Determine the empirical formula of a compound that contains 0.89 g K, 1.18 g Cr, and 1.27 g O. 2. How many atoms are in a 15.0 g sample of magnesium? 3. Find the percentage composition of all elements in sodium sulfate. 4. What is the mass of 6.725 moles of oxygen? 5. A fat is composed of 76.5% carbon, 11.3% oxygen, and 12.2% hydrogen. What is its molecular formula if its molar mass is 847 g/mol? 6. Lithium nitrate occurs as a hydrate with the composition of 56.1% lithium nitrate and 43.9 % water. Calculate the formula of the hydrate. 7. An alkali metal with a mass of .750 grams is found to have 1.15 x 1022 atoms in it. What is the alkali metal? 8. A 10.407 g sample of a hydrate of barium iodide is heated strongly in a crucible to drive off all water of hydration. After heating, the sample has a mass of 9.520 g. Calculate the molecular formula for this hydrate. 9. Determine the percent of carbon in copper phthalocyanine (a green-blue dye) that has the formula Cu(C8H4N2)4. 10. Determine the mass of 8.234 x 1022 formula units of ammonium nitrate. 11. Determine the mass of carbon found in 135 g of silver oxalate. 12. How many oxygen atoms are in 36.2 grams of lead II hydroxide?