composition and hydrates
advertisement

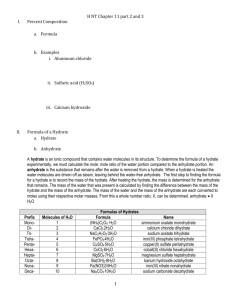
% composition and hydrates What percent of the boxes are orange? Blue? Purple? Yellow? Percentages • Percentage = part / whole • %=p Sales 1st Qtr 2nd Qtr 3rd Qtr 4th Qtr w Think of this like an algebraic equation and you can solve for %, parts, and wholes! KMnO4 • • • • • • • K= 39.10g Mn=54.94g O= 16.00 (4)g Total Mass= 158.04 g % K= 39.10g/158.04g = 24.74 % %Mn=54.94/158.04 =34.76% %O=4(16.00)/158.04 = 40.50% HCl • H= 1.01 g %H= 2.77 % • Cl= 35.45 g %H= 97.23 % • Total Mass= 36.46 g Mg(NO3)2 • Mg= 24.31 g x 1 • N= 14.01 g x 2 • O= 16.00 g x 6 • • • • Total Mass=148.33 g Mg= 24.31 / 148.33 g = 16.39% N= 14.01(2) / 148.33 g =18.89 % O = 16.00(6) / 148.33 = 64.72 % (NH4)3PO4 • • • • • • • • • N= 14.01 g x 3 H= 1.01 g x 12 P= 30.97 g x 1 O= 16.00 g x 4 Total Mass= 149.12 g %N = 14.01(3) / 149.12 g = 28.19% %H= 1.01(12) / 149.12 g =8.13% %P=30.97(1) / 149.12 g =20.77 % %O=16.00 (4) / 149.12 g = 42.92 % Al2(SO4)3 • • • • • • • Al= 26.98g x 2 = S= 32.07g x 3 = O= 16.00g x 12= Total Mass= 342.17 g %Al= 26.98g x 2 /342.17 g =15.77 % %S= 32.07g x 3 / 342.17 g = 28.12 % %O= 16.00g x 12 / 342.17 g = 56.11 % % Water in a hydrate • How is a hydrate different from other compounds? Hydrate anhydrate + H2O • An anhydrate is the chemical component of a hydrate that does not have the water. • A hydrate is a chemical compound that contains water molecules bound to its structure. • Dehydration- a term used to describe a reaction that loses water. Naming • FeCl3-6H2O – Iron (III) Chloride Hexahydrate • CuSO4-5H2O – Copper II Pentahydrate • Barium Chloride Dihydrate – BaCl2-2H2O • Manesium Sulfate Heptahydrate – MgSO4-7H2O Copper II Sulfate Pentahydrate Hydrate Lab Demonstration • http://www.youtube.com/watch?v=5fZlLEBAf EU Naming Practice • FeCl3 6H2O • CuSO4 5H2O Answer: iron (III) chloride hexahydrate Answer: copper II sulfate pentahydrate • Barium Chloride Dihydrate Answer: BaCl2 2H2O • Magnesium Sulfate Heptahydrate Answer: MgSO4 7H2O Percent water in a hydrate • • • • • MgSO4 7H2O Mg= 24.31 S= 32.07 O=4 (16.00) H2O = 7(18.02) % = 7(18.02)g/246.52g • 51.17% Practice • If 125 g of MgSO4 7H2O is completely dehydrated, how many grams of anhydrous magnesium sulfate will remain? – Hint: how many grams of water would there have been driven off? – Hint: what percent of the whole compound is water? – 63.97 g of water, so 61.04 g of anhydrous salt will remain.