Properties of Matter Graphic Organizer_Complete
advertisement

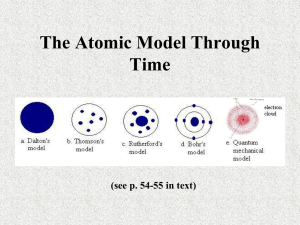
1. Classification of Matter 2. Physical vs. Chemical Properties 3. States of Matter The Heart of the Matter MATTER Mixtures Pure Substances Heterogeneous Elements Homogeneous Compounds Examples: Solute Solution Solvent MATTER Made of Atoms Has Mass & takes up Space Mixtures Pure Substances Made of more than one type of matter Made of one type of matter Elements Made of one type of atom Atom + Atom = Element Compounds Made of 2 or More atoms bonded together Atom + Atom = Molecule Molecule + Molecule = Compound Heterogeneous Made of more than one phase Can be separated Examples: Trail Mix Salad Solute The stuff that is dissolved In the liquid Ex: Kool-Aid mix Homogeneous one phase Solution All its regions are Identical Ex: Kool-Aid Solvent The liquid that forms the solution Ex: Water Properties of Matter States Solid Liquid Gas Plasma Changes of State Melting Freezing Boiling Condensation Ionization Relaxation Physical Properties Describe a Substance Chemical Properties Describe how it can form a new substance Signs of Change Characteristics Color Shape Size Texture Mass Volume Density Odor Change in Temp Change in Color Bubbles form Solids form Examples Burning Rusting Rotting Chemical Reactions Atoms in two substances combine to form new substances Examples 2Na+Cl22NaCl 2H2+O22H2O States of Matter Plasma Temperature ________Energy Atoms ____stuck together Gas ______Energy Atoms _____stuck together _______Point ______Point Liquid _________Point Solid _____Energy Atoms ______stuck together ____________Energy Atoms __________stuck together _________Point States of Matter Plasma Temperature Highest Energy Atoms not stuck together Gas High Energy Atoms not stuck together Boiling Point Dew Point Liquid _Melting Point Solid Low Energy Atoms tightly stuck together Medium Energy Atoms loosely stuck together Freezing Point 1. 2. 3. 4. 5. 6. 7. 8. Molecule____ Element ____ Compound ____ Volume ____ Mixture ____ Atom ____ Mass ____ Matter ____ A. Amount of matter in an object B. The smallest building block of matter C. 2 or more atoms combined together D. The amount of space an object occupies E. Is made of atoms F. A substance with only 1 type of atom G. A substance with 2 or more atoms bound together H. A combination of different substances which retain their individual properties 1. 2. 3. 4. 5. 6. 7. 8. Molecule__C__ Element __F__ Compound _G__ Volume _D___ Mixture __H__ Atom __B__ Mass __A__ Matter __E__ A. Amount of matter in an object B. The smallest building block of matter C. 2 or more atoms combined together D. The amount of space an object occupies E. Is made of atoms F. A substance with only 1 type of atom G. A substance with 2 or more atoms bound together H. A combination of different substances which retain their individual properties