Chapter 6 Notes
advertisement

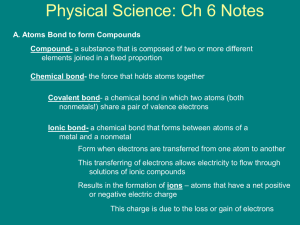
Chapter 6 Notes Chemical Bonding Section 1:Introduction to Chemical Bonding Atoms seldom exist as independent particles in nature. Most substances consist of combinations of atoms held together by chemical bonds. Chemical Bond – A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together. Section 1:Introduction to Chemical Bonding You might ask why most particles bond to others? This is because as independent particles most atoms have relatively high potential energies. Nature, however, favors arrangements in which potential energy is minimized. This means that most atoms are less stable existing by themselves than when they are combined. By bonding with each other, atoms decrease their potential energy, thereby creating more stable arrangements. Section 1:Introduction to Chemical Bonding Types of Chemical Bonding When atoms bond, their valence electrons are redistributed in a way that makes the atoms more stable. The manner in which the electrons are redistributed determines what type of bond will be made. Section 1:Introduction to Chemical Bonding Ionic Bonding – Chemical bonding that results from the electrical attraction between cations and anions. In purely ionic bonds, an atom will give up electrons to another atom in the compound. In contrast, a covalent bond is made when the atoms share electrons. Covalent Bonding – Chemical bonding that results from the sharing of valence electrons between atoms. Section 1:Introduction to Chemical Bonding Bonding between atoms is rarely purely ionic or covalent. It usually falls somewhere in the middle depending upon how strongly the atoms are attracted to the electrons of the other atoms in the compound. Section 1:Introduction to Chemical Bonding Remember that electronegativity is a measure of an atom’s ability to attract electrons. The degree to which bonding between atoms of two elements is ionic or covalent can be estimated by calculating the difference in the element’s electronegativities. Section 1:Introduction to Chemical Bonding Ionic – any difference that is greater than 1.7 Polar-Covalent – any difference that is greater than or equal to .3 and less than or equal to 1.7 Non-polar covalent – any difference that is less than .3 Section 1:Introduction to Chemical Bonding Non-polar covalent bond – A covalent bond in which the bonding electrons are shared equally by the bonding atoms, resulting in a balanced distribution of electrical charge. Polar – An uneven distribution of charge. Polar-covalent bond – A covalent bond in which the bonded atoms have an unequal attraction for the shared electrons. Section 1:Introduction to Chemical Bonding Hydrogen is an element that is never found alone. Even when it is just an element, hydrogen is at least bonded to itself as H2. In this sort of situation the electronegativities between the two elements in the compound would be 0. What that means is that the electrons that are shared between the two atoms would be shared equally because the atoms are pulling on the electrons with the same force. Section 1:Introduction to Chemical Bonding However, in water, hydrogen is bonded to a different atom. Oxygen has a significantly higher electronegativity than hydrogen does. Therefore, the oxygen nucleus pulls harder on the electrons that are being shared. The electrons are still shared, just not equally. Section 1:Introduction to Chemical Bonding They are more likely to be found around the oxygen nucleus than the hydrogen nucleus because oxygen pulls harder on them. That, in turn, makes the water molecule polar. All polar means is that it has a positive side and a negative side. Because the electrons spend more time toward the oxygen, that side is slightly negative and the hydrogens are slightly positive. Section 2:Covalent Bonding and Molecular Compounds Many chemical compounds found in living things, and produced by living things, are composed of molecules. Molecule – A neutral group of atoms that are held together by covalent bonds. Molecular compound – A chemical compound whose simplest units are molecules. Section 2:Covalent Bonding and Molecular Compounds A single molecule of a chemical compound is an individual unit capable of existing on its own. It may consist of two or more atoms of the same element, such as H2, or of two or more different atoms as in CO2. Section 2:Covalent Bonding and Molecular Compounds The composition of a compound is given by its chemical formula. Chemical formula – Indicates the relative numbers of atoms of each kind in a chemical compound by using atomic symbols and numerical subscripts. We use the term chemical formula to describe any compound. If we are specifically talking about a covalent compound, however, we would refer to it as a molecular formula. Section 2:Covalent Bonding and Molecular Compounds Molecular formula – Shows the types and numbers of atoms combined in a single molecule of a molecular compound. The molecular formula for water, for example, would be H2O. This reflects the fact that there are two hydrogen atoms that are covalently bonded to an oxygen atom. Section 2:Covalent Bonding and Molecular Compounds Formation of a Covalent Bond So how does a covalent bond form? Well, remember that we said that all atoms have a certain energy associated with them? When those atoms are bonded, usually it lowers the amount of potential energy that they have. Keep this in mind. Section 2:Covalent Bonding and Molecular Compounds Picture two isolated hydrogen atoms separated by a distance large enough that they do not influence each other. At this distance, the overall potential energy of them is arbitrarily set at 0. Now, consider what must happen as those two atoms approach each other. The electrons will repel each other and the nuclei will too. Section 2:Covalent Bonding and Molecular Compounds However, the electron from one hydrogen atom will be attracted to the other hydrogen nucleus. As the get closer to each other the potential energy must be decreasing. At some point, the nuclei will be at the optimum distance from each other, shown by the lowest possible potential energy for those two atoms. Section 2:Covalent Bonding and Molecular Compounds As you continue to push the atoms together, the potential energy will increase because the nuclei are too close to each other and they want to repel. Like pushing two positive ends of magnets together. Section 2:Covalent Bonding and Molecular Compounds Section 2:Covalent Bonding and Molecular Compounds The relative strength of attraction and repulsion between the charged particles depends on the distance separating the atoms. When the atoms first begin to interact, the electron-proton attraction is stronger than the electron-electron and proton-proton repulsion. Section 2:Covalent Bonding and Molecular Compounds As they get closer, eventually there is a distance where that ratio shifts the other direction. At the point where those two forces equal each other is where the atoms are at the lowest potential energy Section 2:Covalent Bonding and Molecular Compounds Characteristics of the Covalent Bond The electrons of each hydrogen atom of the hydrogen molecule are shared between the nuclei. They can be pictured as occupying overlapping orbitals, moving about freely in either orbital. The bonded atoms vibrate a bit, but as long as their potential energy stays close to the minimum, they will remain covalently bonded. Section 2:Covalent Bonding and Molecular Compounds This distance between two bonded atoms at their minimum potential energy is known as the bond length. The bond length between two hydrogen atoms is 75 pm. We saw earlier that the hydrogen atoms lowered their energy to form H2. That same amount of energy that was released to form the hydrogen molecule would be required in order to break the bond as well. Section 2:Covalent Bonding and Molecular Compounds Bond energy – the energy required to break a chemical bond and form neutral isolated atoms. The energy relationships described here for the formation of a hydrogen-hydrogen bond apply generally to all covalent bonds. However, bond lengths and bond energies differ depending upon the atoms in the bond. Section 2:Covalent Bonding and Molecular Compounds The Octet Rule Unlike other atoms, the noble gas atoms exist independently in nature. They possess a minimum of energy existing on their own because of the special stability of their electron configurations. Their stability comes from the fact that their outer energy level is full of electrons. Other atoms can effectively fill their valence shell by sharing electrons through covalent bonding. Such bond formation follows the octet rule. Section 2:Covalent Bonding and Molecular Compounds Octet rule – Chemical compounds tend to form so that each atom, by gaining, losing, or sharing electrons, has an octet of electrons in its highest occupied energy level. Section 2:Covalent Bonding and Molecular Compounds Electron Dot Notation Covalent bond formation usually involves only the electrons in an atom’s valence shell. To keep track of these electrons it is helpful to use electron dot notation. Electron-dot notation – An electron configuration notation in which only the valence electrons of an atom of a particular element are shown, indicated by dots placed around the element’s symbol. Section 2:Covalent Bonding and Molecular Compounds Section 2:Covalent Bonding and Molecular Compounds Lewis Structures Electron dot notation can also be used to represent molecules. For example, a hydrogen molecule, H2, is represented by combining the notations of two individual hydrogen atoms, as follows: The pair of dots represents the shared electron pair of the hydrogen-hydrogen covalent bond. Section 2:Covalent Bonding and Molecular Compounds When we use fluorine, you can see the electrons that are involved in the covalent bond. You can also see the unshared pairs of electrons that are surrounding the fluorine nuclei. The shared electrons most often are replaced with a dash to represent the bond. Section 2:Covalent Bonding and Molecular Compounds These representations are known as Lewis structures. Lewis structures – Formulas in which atomic symbols represent nuclei and inner shell electrons, dot pairs or dashes between two atomic symbols represent electron pairs in covalent bonds, and dots adjacent to only one atomic symbol represent unshared electrons. Section 2:Covalent Bonding and Molecular Compounds It is common to write Lewis structures that only represent the electrons that are involved in bonding. This is called structural formulas. Structural formula – indicates the kind, number, arrangement, and bonds but not the unshared pairs of electrons in the atom. Single bond – a covalent bond in which one pair of electrons is shared between two atoms. Section 2:Covalent Bonding and Molecular Compounds Multiple Covalent Bonds Atoms of some elements can share more than one electron pair. A double covalent bond, or more simply a double bond, is a bond in which two pairs of electrons are shared between two atoms. You can either show this by having two pairs of dots or two lines. Section 2:Covalent Bonding and Molecular Compounds Section 2:Covalent Bonding and Molecular Compounds A triple bond, therefore, would be the sharing of three pairs of electrons between two atoms. You would represent this by 6 dots or 3 lines. Multiple bonds – double or triple bonds Section 2:Covalent Bonding and Molecular Compounds Resonance Structures Some molecules and ions cannot be represented adequately by a single Lewis structure. One such molecule is ozone, O3. Section 2:Covalent Bonding and Molecular Compounds Notice that each structure indicates that the ozone molecule has two types of bonds, one single and one double. Scientists used to think that ozone split its time between these two states, resonating back and forth between having a single and double bond. However, experiments have shown that the bonds in ozone are identical. Therefore scientists now say that ozone has a single structure that is the average of these two structures. Section 2:Covalent Bonding and Molecular Compounds Resonance – Refers to bonding in molecules or ions that cannot be correctly represented by a single Lewis structure. Section 3: Ionic Bonding and Ionic Compounds Most of the rocks and minerals that make up Earth’s crust consist of positive and negative ions held together by ionic bonding. A familiar ionic compound is sodium chloride, table salt. A sodium ion, Na+, has a charge of 1+. A chloride ion, Cl -, has a charge of -1. Section 3: Ionic Bonding and Ionic Compounds There is an electrical force of attraction between oppositely charged ions. In ionic compounds, the atoms combine in such a way as to cancel out the charges that exist on the ions. For this example, it will take one sodium ion to cancel out the charge of one chloride ion. Section 3: Ionic Bonding and Ionic Compounds Ionic compound – Compound composed of positive and negative ions that are combined so that the numbers of positive and negative charges are equal. Most ionic compounds exist as a 3 dimensional network of positive and negative ions. Not as individual molecules like covalent compounds. Section 3: Ionic Bonding and Ionic Compounds Therefore, the chemical formula of the ionic compound does not represent individual, neutral units, but rather, the simplest ratio of the compound’s combined ions that gives electrical neutrality. Formula unit – The simplest collection of atoms from which an ionic compound’s formula can be established. Section 3: Ionic Bonding and Ionic Compounds The ratio of ions in a formula unit depends upon the charges of the ions combined. For example, if sodium combined with a fluoride ion, that would be a one to one ratio because they both have a magnitude of 1 in their charge. However, if magnesium combined with fluoride, it would take two fluorides to balance out one magnesium because the charge on a magnesium ion is 2+. Section 3: Ionic Bonding and Ionic Compounds When the sodium atom comes into contact with the chlorine atom, the difference in electronegativity is very large. Chlorine rips an electron from the sodium atom, thus causing the atom to become a positive ion. With the gaining of another electron, chlorine becomes a chloride ion. Now that we have ions, they are electrically attracted to one another and an ionic bond is formed. Section 3: Ionic Bonding and Ionic Compounds Formation of Ionic Compounds Electron dot notation can be used to demonstrate the changes that take place in ionic bonding. Ionic compounds do not ordinarily form from the combination of isolated ions, but let’s pretend they do for a minute. This will be easier to understand. Section 3: Ionic Bonding and Ionic Compounds Characteristics of Ionic Bonding Remember that nature works to lower the potential energies of the atoms involved in bonding. In an ionic crystal, ions minimize their potential energy by combining in an orderly arrangement known as a crystal lattice. Section 3: Ionic Bonding and Ionic Compounds Section 3: Ionic Bonding and Ionic Compounds The attractive forces at work within an ionic crystal include those between oppositely charged ions and those between the nuclei and electrons of adjacent ions. The repulsive forces include those between like charged ions and those between electrons of adjacent ions. The placement of ions in a crystal lattice represents a balance of these forces. Section 3: Ionic Bonding and Ionic Compounds To compare bond strengths in ionic compounds, chemists compare the amounts of energy released when separated ions in a gas come together to form a crystalline solid. Lattice energy – The energy released when one mole of an ionic crystalline compound is formed from gaseous ions. Section 3: Ionic Bonding and Ionic Compounds A Comparison of Ionic and Molecular Compounds The force that holds ions together in ionic compounds is a very strong overall attraction between positive and negative charges. In a molecular compound, the covalent bonds of the atoms making up each molecule are also strong. Section 3: Ionic Bonding and Ionic Compounds However, the forces holding the individual molecules to other molecules is not. This difference in strength of attraction for molecules and formula units gives rise to several interesting properties. Section 3: Ionic Bonding and Ionic Compounds The melting point, boiling point, and hardness of a compound all are based on how strongly the base units are attracted to each other. Because the forces between individual molecules is not very strong, covalent compounds tend to have lower boiling and melting points than ionic compounds. Section 3: Ionic Bonding and Ionic Compounds In fact, many covalent compounds are already a gas at room temperature. Ionic compounds tend to have very high melting and boiling points because of the strong attractiveness between units. Section 3: Ionic Bonding and Ionic Compounds Ionic compounds are hard, but brittle. Think about it. You basically have several sheets of ions all stacked upon each other. They are held in place because of all of the forces acting upon them. If one of those sheets gets shifted by just a small amount, then all of those forces are lined up in the total opposite direction. Section 3: Ionic Bonding and Ionic Compounds This would bring about massive repulsive charges. That sheet of ions has now been split from the others. This is why when an ionic compound breaks, it does so very cleanly. Section 3: Ionic Bonding and Ionic Compounds In their solid state, ionic compounds cannot conduct electricity. However, if the compound can be dissolved, it will conduct electricity because the compounds break up into charged particles. Covalent compounds usually do not conduct electricity in either case. Even when the compound dissolves, they just break up into separate molecules, not charges. Section 3: Ionic Bonding and Ionic Compounds Polyatomic Ions Some atoms bond covalently with each other to form a group of atoms that has both molecular and ionic characteristics. Polyatomic ions – A charged group of covalently bonded atoms. Polyatomic ions bond with other ions to form ionic compounds. The charge on a polyatomic ion comes from having too many or too few electrons. Section 4: Metallic Bonding Chemical bonding is different in metals than it is in ionic and covalent. For one thing, they are great conductors of electricity, even better than molten ionic compounds. This is due to the highly mobile valence electrons of the metals. Section 4: Metallic Bonding The Metallic Bond Model The highest energy levels of most metals are occupied by very few electrons. This leaves many orbitals vacant. All of these vacant orbitals in the outer energy levels overlap with the atom next to them. Section 4: Metallic Bonding The overlapping of empty orbitals allows the valence electrons of metals to be delocalized. That means that they do not belong to any one atom of metal. It is sort of thought of as a sea of electrons that have metal atoms packed together in a crystal lattice. Metallic Bonding – the chemical bonding that results from the attraction between metal atoms and the surrounding sea of electrons. Section 4: Metallic Bonding Metallic Properties The freedom of electrons to move in a network of metal atoms accounts for the high electrical and thermal conductivity characteristic of all metals. Because they contain so many orbitals that are separated by only a small amount of energy, they are able to absorb a wide range of light frequencies. Section 4: Metallic Bonding Those electrons will then become excited and move to a higher energy level. They cannot hold onto that extra energy and will release it in the form of light at a frequency similar to the one they absorbed. This is what gives metals the shine or luster that we are familiar with. Most metals are easily formed into desired shapes. Section 4: Metallic Bonding Malleability – ability of a substance to be hammered or beaten into thin sheets. Ductility – the ability of a substance to be drawn, pulled, or extruded through a small opening to produce a wire. Section 4: Metallic Bonding These properties are possible in metals because in their lattice, all of the bonding is the same in all directions. Unlike ionic crystals with their build up of repulsive charges when one sheet is moved, metals have no such build up. Therefore, the atoms can slide past each other and take another form. Section 5: Molecular Geometry The properties of molecules depend not only on the bonding of atoms but also on molecular geometry. Atoms are not flat, they have depth. They are 3 dimensional particles. Molecular geometry is the 3 dimensional arrangements of a molecule’s atoms. Section 5: Molecular Geometry A chemical formula reveals very little about a molecule’s geometry. After many tests designed to reveal the shapes of various molecules, chemists developed two different theories to explain certain aspects of their findings. One theory accounts for molecular bond angles. The other is used to describe the orbitals that contain the valence electrons of a molecule’s atoms. Section 5: Molecular Geometry VSEPR Theory Diatomic molecules, like those of H2 and HCl, must be linear because only one bond has been made. However, to predict the geometries of more complicated molecules you must consider the locations of all electron pairs surrounding the bonded atoms. This is the basis of the VSEPR theory. Section 5: Molecular Geometry VSEPR stands for “valence shell electron pair repulsion.” This refers to the repulsion between pairs of valence electrons in the atoms of a molecule. VSEPR Theory – repulsion between the sets of valence-level electrons surrounding an atom causes these sets to be oriented as far apart as possible. Section 5: Molecular Geometry Let’s examine the simple molecule BeF2. The beryllium atom forms a covalent bond with each fluorine atom. According to VSEPR theory, the shared pairs will be as far away from each other as possible. With atoms being three dimensional, the farthest that two electron pairs can be from each other is 180o. Thus all three atoms would be in a straight line, the molecule would be linear. Draw BeF2: Section 5: Molecular Geometry If we allow the central atom to be represented by the letter A and we represent the atoms bonded to the central atom as B, then this would be an example of a AB2 molecule. All AB2 molecules are linear. What would you expect an AB3 molecule to look like? How many bonds to the central atom? What degree would the bonds be from each other? Draw BF3: Section 5: Molecular Geometry This is an AB3 molecule. The three bonds stay as far apart as possible by being 120o away from each other. This would be called trigonal planar. An AB4 molecule would have four bonds to the central atom. Most people automatically think that the bonds would be 90o apart. However, because the atoms are 3 dimensional, the bonds can actually be 109.5o apart. This shape is called a tetrahedral. Draw CH4: Section 5: Molecular Geometry Practice: Use VSEPR to predict the molecular geometry of the following molecules: HI CBr4 CH2Cl2 Section 5: Molecular Geometry VSEPR Theory and Unshared Electron Pairs Ammonia, NH3, and water, H2O, are examples of molecules in which the central atom has both shared and unshared electron pairs. Draw Lewis Structure of Ammonia: Section 5: Molecular Geometry As you can see, ammonia has three hydrogen atoms bonded to a central nitrogen atom. The nitrogen atom has an unshared pair of electrons as well. VSEPR theory says that the lone pair occupies space around the nitrogen atom just as the bonding pairs do. Therefore, this isn’t really an AB3 molecule, it is an AB3E molecule. Section 5: Molecular Geometry We would not call this a tetrahedral molecule, however. Our description of shapes of molecules only refers to the positions of atoms. Therefore, this would look like a trigonal planar molecule that has been bent downward a little. This is called trigonal pyramidal. Draw Ammonia: Section 5: Molecular Geometry Water has two unshared electron pairs. It is an AB2E2 molecule. The oxygen atom can be thought of as being in the center of a tetrahedron, with two corners occupied by unshared electron pairs. Remember that the shape is only determined by the atoms, not the unshared pairs. Therefore, this is a bent molecule. Section 5: Molecular Geometry Draw Water: Not a big deal, but unshared electron pairs repel more than a regular bond. Therefore, trigonal pyramidal and bent bonds do not have 109.5o bonds like tetrahedral, but a little smaller. Finally in VSEPR, double and triple bonds are treated just as a single bond. Polyatomic ions are treated similarly to molecules. Use this table to help you predict the geometries for other types of molecules. Section 5: Molecular Geometry Section 5: Molecular Geometry Section 5: Molecular Geometry Hybridization VSEPR theory is useful for explaining the shapes of molecules. However, it does not reveal the relationship between a molecule’s geometry and the orbitals occupied by its bonding electrons. We use a different model to explain how the orbitals of an atom become rearranged when the atom forms covalent bonds. This model is called hybridization. Section 5: Molecular Geometry Hybridization – The mixing of two or more atomic orbitals of similar energies on the same atom to produce new hybrid atomic orbitals of equal energies. We will practice with methane, CH4. The orbital notation for a carbon atom is as shown below. Section 5: Molecular Geometry We know from earlier that methane has a tetrahedral shape. How does carbon form four equivalent, tetrahedrally arranged covalent bonds by orbital overlap with four other atoms? Two of carbon’s valence electrons occupy the 2s orbital and two occupy the 2p orbital. Remember that 2s and 2p orbitals have different shapes. Section 5: Molecular Geometry To achieve four equivalent bonds, carbon’s 2s and three 2p orbitals hybridize to form four new, identical orbitals called sp3 orbitals. The superscript 3 indicates that three p orbitals were included in the hybridization. The superscript 1 on the s is understood. The sp3 orbitals all have the same energy, which is more than a 2s, but less than a 2p. Section 5: Molecular Geometry Draw carbon’s orbital notation with increasing energy: Hybrid orbitals – Orbitals of equal energy produced by the combination of two or more orbitals on the same atom. Section 5: Molecular Geometry The number of hybrid orbitals produced equals the number of orbitals that have been combined. Hybridization also explains the bonding and geometry of many molecules formed by group 15 and 16 elements. The sp3 hybridization of a nitrogen atom yields four hybrid orbitals. Three that contain an unpaired electron and one that contains an unshared pair of electrons. Section 5: Molecular Geometry Each unpaired electron is capable of forming a bond. Similarly, two of the four sp3 hybrid orbitals on an oxygen atom are occupied by two electron pairs and two are occupied by unpaired electrons. Each unpaired electron can form a single bond. Section 5: Molecular Geometry The linear geometry of BeF2 is made possible by hybridization involving the s orbital and one available empty p orbital to yield sp hybrid orbitals. The trigonal planar geometry of BF3 is made possible by hybridization involving the s orbital and 2 of the p orbitals to make sp2 hybrid orbitals. Use the following table to help with recognizing hybridization . Section 5: Molecular Geometry Section 5: Molecular Geometry Intermolecular Forces As a liquid is heated, the kinetic energy of its particles increases. At the boiling point, the energy is enough that the force of attraction between the liquid’s particles is overcome. The particles will pull away from each other and enter into the gas phase. Boiling point is a great measure of the force of attraction between particles of a liquid. The higher the boiling point is, the stronger the forces between the particles must have been. Section 5: Molecular Geometry The forces of attraction is known as intermolecular forces. Intermolecular forces, IMF, can vary in strength, but are usually weaker than covalent, ionic, and metallic bonds. Molecular Polarity and Dipole-Dipole Forces The strongest IMF exist between polar molecules. They act as tiny dipoles because of their uneven charge distribution. Dipole – IMF created by equal but opposite charges that are separated by a short distance. Section 5: Molecular Geometry The negative region in one polar molecule attracts the positive region in adjacent molecules, and so on throughout a liquid or solid. The forces of interaction between polar molecules are known as dipoledipole forces. Molecules that display this type of IMF will show a much higher melting and boiling point because of the extra attractions. Section 5: Molecular Geometry Polar molecules can also induce a dipole moment in other molecules that usually do not exhibit such behavior. For instance, water can cause O2 to temporarily display dipole behavior because it is highly polar. However, the induced dipole is temporary and very weak. Section 5: Molecular Geometry Hydrogen Bonding Some hydrogen containing molecules exhibit very high boiling points because of an unusually strong type of dipole-dipole force known as hydrogen bonding. We see hydrogen bonding in such molecules as water, ammonia, and hydrogen fluoride. In each of these molecules, hydrogen is bonded with an atom that is very electronegative. Section 5: Molecular Geometry This causes the electrons to spend more time around the central atom than hydrogen. This leaves a partial positive charge on what is essentially a proton hanging off of another atom. Because there are no other electrons on the hydrogen, it can get very close to other molecules. This creates a very strong IMF. Section 5: Molecular Geometry Hydrogen Bonding – The IMF in which a hydrogen atom that is bonded to a highly electronegative atom is attracted to an unshared pair of electrons of an electronegative atom in a nearby molecule. Section 5: Molecular Geometry London Dispersion Forces Even noble-gas atoms and molecules that are nonpolar experience a weak IMF. Electrons are in constant motion. At any moment the electrons could overload one side of the atom just by chance. This causes a very temporary dipole moment. The molecule or atom would then attract another molecule or atom that is close to it. Section 5: Molecular Geometry London Dispersion Forces – The IMF attractions resulting from the constant motion of electrons and the creation of instantaneous dipoles. These type of IMF can occur in any molecule, but they are the only IMF that occurs when you have noble gas atoms.