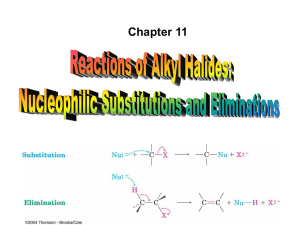
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and
advertisement

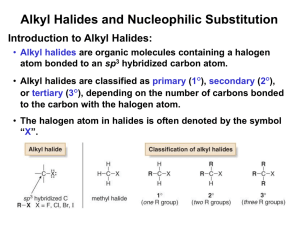
REAKSI ALKIL HALIDA: SUBSTITUSI DAN ELIMINASI NUKLEOFILIK Nukleofil dan gugus pergi: Reaksi alkil halida dengan nukleofil Alkil halida terpolarisasi pada ikatan karbonhalida, membuat karbon menjadi elektrofil. Nukleofil mengganti halida pada ikatan C-X (sebagai basa Lewis) Nukleofil yang memeiliki basa Brønsted kuat dapat menghasilkan produk eliminasi. Nukleofil Basa Lewis yang netral atau bermuatan negatif Perubahan muatan pada reaksi nukleofil Nukleofil netral menjadi bermuatan positif Nukleofil bermuatan negatif menjadi netral Based on McMurry, Organic Chemistry, 6th edition, (c) 2003 4 Reaktifitas Relatif Nukleofil Tergantung pada kondisi reaksi Nukleofil dengan sifat basa lebih kuat bereaksi lebih cepat untuk struktur yang sama. Nukleofil yang baik terletak lebih bawah dalam SPU. Anion biasanya lebih reaktif dari yang netral. Based on McMurry, Organic Chemistry, 6th edition, (c) 2003 5 Based on McMurry, Organic Chemistry, 6th edition, (c) 2003 6 Gugus Pergi A good leaving group reduces the barrier to a reaction Stable anions that are weak bases are usually excellent leaving groups and can delocalize charge Based on McMurry, Organic Chemistry, 6th edition, (c) 2003 7 “Super” Leaving Groups Based on McMurry, Organic Chemistry, 6th edition, (c) 2003 8 Poor Leaving Groups If a group is very basic or very small, it is prevents reaction Based on McMurry, Organic Chemistry, 6th edition, (c) 2003 9 Reaction Kinetics The study of rates of reactions is called kinetics The order of a reaction is sum of the exponents of the concentrations in the rate law – the first example is first order, the second one second order. C H3 N aO H + C H3 C C H3 Br N aBr + C H3 C H3 + C H 3B r v = k [C H 3 B r][N a O H ] OH C H3 v = k [C 4 H 9 B r] N aO H C N aBr + C H 3O H The SN1 and SN2 Reactions Follow first or second order reaction kinetics Ingold nomenclature to describe characteristic step: S=substitution N (subscript) = nucleophilic 1 = substrate in characteristic step (unimolecular) 2 = both nucleophile and substrate in characteristic step (bimolecular) Stereochemical Modes of Substitution Substitution with inversion: Substitution with retention: Substitution with racemization: 50% - 50% SN2 Process The reaction involves a transition state in which both reactants are together “Walden” Inversion Keadaan Transisi SN2 Keadaan transisi reaksi SN2 adalah planar, karbon mengikat tiga gugus. Urutan Kereaktifan Reaksi SN2 Semakin banyak gugus alkil terikat reaksi semakin lambat Based on McMurry, Organic Chemistry, 6th edition, (c) 2003 16 Pengaruh sterik pada Reaksi SN2 The carbon atom in (a) bromomethane is readily accessible resulting in a fast SN2 reaction. The carbon atoms in (b) bromoethane (primary), (c) 2-bromopropane (secondary), and (d) 2-bromo-2-methylpropane (tertiary) are successively more hindered, resulting in successively slower SN2 reactions. Steric Hindrance Raises Transition State Energy Very hindered Steric effects destabilize transition states Severe steric effects can also destabilize ground state 11.5 Characteristics of the SN2 Reaction Sensitive to steric effects Methyl halides are most reactive Primary are next most reactive Secondary might react Tertiary are unreactive by this path No reaction at C=C (vinyl halides) The SN1 Reaction Tertiary alkyl halides react rapidly in protic solvents by a mechanism that involves departure of the leaving group prior to addition of the nucleophile Called an SN1 reaction – occurs in two distinct steps while SN2 occurs with both events in same step Stereochemistry of SN1 Reaction The planar intermediate leads to loss of chirality A free carbocation is achiral Product is racemic or has some inversion SN1dalam Kenyataannya Karbokation cenderung bereaksi pada sisi yang berlawanan dari gugus pergi lepas Suggests reaction occurs with carbocation loosely associated with leaving group during nucleophilic addition Effects of Ion Pair Formation If leaving group remains associated, then product has more inversion than retention Product is only partially racemic with more inversion than retention Associated carbocation and leaving group is an ion pair SN1 Energy Diagram k1 k-1 k2 Step through highest energy point is rate-limiting (k1 in forward direction) V = k[RX] Rate-determining step is formation of carbocation 11.9 Characteristics of the SN1 Reaction Tertiary alkyl halide is most reactive by this mechanism Controlled by stability of carbocation Delocalized Carbocations Delocalization of cationic charge enhances stability Primary allyl is more stable than primary alkyl Primary benzyl is more stable than allyl Perbandingan : Mekanisme Substitusi SN1 Dua tahap dengan hasil antara karbokation Terjadi pada 3°, allil, benzil SN2 Satu tahap tanpa hasil antara Terjadi pada alkil halida primer dan sekunder Effect of Leaving Group on SN1 Critically dependent on leaving group Reactivity: the larger halides ions are better leaving groups In acid, OH of an alcohol is protonated and leaving group is H2O, which is still less reactive than halide p-Toluensulfonate (TosO-) is excellent leaving group Based on McMurry, Organic Chemistry, 6th edition, (c) 2003 28 Allylic and Benzylic Halides Allylic and benzylic intermediates stabilized by delocalization of charge (See Figure 11-13) Primary allylic and benzylic are also more reactive in the SN2 mechanism Based on McMurry, Organic Chemistry, 6th edition, (c) 2003 29 Based on McMurry, Organic Chemistry, 6th edition, (c) 2003 30 The Solvent Solvents that can donate hydrogen bonds (-OH or –NH) slow SN2 reactions by associating with reactants Energy is required to break interactions between reactant and solvent Polar aprotic solvents (no NH, OH, SH) form weaker interactions with substrate and permit faster reaction Based on McMurry, Organic Chemistry, 6th edition, (c) 2003 31 Based on McMurry, Organic Chemistry, 6th edition, (c) 2003 32 Polar Solvents Promote Ionization Polar, protic and unreactive Lewis base solvents facilitate formation of R+ Solvent polarity is measured as dielectric polarization (P) Solvent Is Critical in SN1 Stabilizing carbocation also stabilizes associated transition state and controls rate Solvation of a carbocation by water Effects of Solvent on Energies Polar solvent stabilizes transition state and intermediate more than reactant and product Polar aprotic solvents Form dipoles that have well localized negative sides, poorly defined positive sides. Examples: DMSO, HMPA (shown here) O P C H3 N N C H3 N C H3 C H3 C H C H3 3 + + + Common polar aprotic solvents O S C H3 C H3 d im e th y lsu lfo xid e (D M S O ) O P C H3 N N C H3 N C H3 C H3 C H3 C H3 h e xa m e th ylp h o s p h o ra m id e (H M P A ) O H C N C H3 N ,N -d im e th y lfo rm a m id e (D M F ) C H3 s u lfo la n e S O O Polar aprotic solvents solvate cations well, anions poorly + + + - - + + + + + + + + + - good fit! Cl bad fit! - Na - - + + + - + + + + + + + + + + SN1: Carbocation not very encumbered, but needs to be solvated in rate determining step (slow) Polar protic solvents are good because they solvate both the leaving group and the carbocation in the rate determining step k1! The rate k2 is somewhat reduced if the nucleophile is highly solvated, but this doesn’t matter since k2 is inherently fast and not rate determining. SN2: Things get tight if highly solvated nucleophile tries to form pentacoordiante transition state Polar aprotic solvents favored! There is no carbocation to be solvated. Nucleophiles in SN1 Since nucleophilic addition occurs after formation of carbocation, reaction rate is not affected normally affected by nature or concentration of nucleophile REAKSI ELIMINASI ALKIL HALIDA Eliminasi merupakan salah satu jalan alternatif dari suatu reaksi substitusi Lawan dari reaksi adisi Menghasilkan alkena Menurunkan produk substitusi terutama SN1 Aturan Zaitsev’s untuk Reaksi Eliminasi (1875) Pada eliminasi HX dari suatu alkil halida, produk tersubstitusi lebih dominan Mechanisms of Elimination Reactions Ingold nomenclature: E – “elimination” E1: X- leaves first to generate a carbocation a base abstracts a proton from the carbocation E2: Concerted transfer of a proton to a base and departure of leaving group 11.11 The E2 Reaction Mechanism A proton is transferred to base as leaving group begins to depart Transition state combines leaving of X and transfer of H Product alkene forms stereospecifically Geometry of Elimination – E2 Antiperiplanar allows orbital overlap and minimizes steric interactions E2 Stereochemistry Overlap of the developing orbital in the transition state requires periplanar geometry, anti arrangement Allows orbital overlap Predicting Product E2 is stereospecific Meso-1,2-dibromo-1,2-diphenylethane with base gives cis 1,2-diphenyl RR or SS 1,2-dibromo-1,2-diphenylethane gives trans 1,2-diphenyl (E)-1bromo-1,2-diphenylethene 11.12 Elimination From Cyclohexanes Abstracted proton and leaving group should align trans-diaxial to be anti periplanar (app) in approaching transition state (see Figures 11-19 and 11-20) Equatorial groups are not in proper alignment 11.14 The E1 Reaction Competes with SN1 and E2 at 3° centers V = k [RX] Stereochemistry of E1 Reactions E1 is not stereospecific and there is no requirement for alignment Product has Zaitsev orientation because step that controls product is loss of proton after formation of carbocation Comparing E1 and E2 Strong base is needed for E2 but not for E1 E2 is stereospecifc, E1 is not E1 gives Zaitsev orientation 11.15 Summary of Reactivity: SN1, SN2, E1, E2 Alkyl halides undergo different reactions in competition, depending on the reacting molecule and the conditions Based on patterns, we can predict likely outcomes Special cases, both SN1 and SN2 blocked (or exceedingly slow) Br Carbocation highly unstable, attack from behind blocked Carbocation can’t flatten out as required by sp2 hybridization, attack from behind blocked Also: elimination not possible, can’t place double bond at bridgehead in small cages (“Bredt’s rule”) Br Carbocation highly unstable, attack from behind blocked Br C H3 C H2B r C H3 C H3 Carbocation would be primary, attack from behind difficult due to steric blockage Kinetic Isotope Effect Substitute deuterium for hydrogen at position Effect on rate is kinetic isotope effect (kH/kD = deuterium isotope effect) Rate is reduced in E2 reaction Heavier isotope bond is slower to break Shows C-H bond is broken in or before ratelimiting step