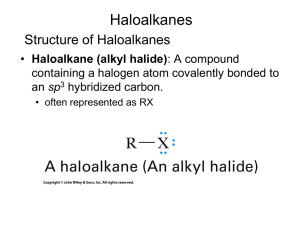Practice Problem
advertisement

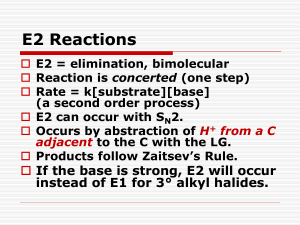
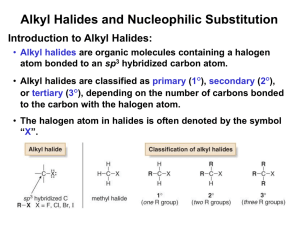
Chapter 11 Introduction • Alkyl halides – are polarized at the carbonhalide bond, making the carbon electrophilic – are electrophiles • Alkyl halides – react with nucleophiles and bases – undergo substitution of X by Nu – undergo elimination of HX to yield an alkene • Substitution: Nucleophiles will replace the X in C-X bonds (act as Lewis bases) • Elimination: Nucleophiles that are Brønsted bases produce elimination A. The Discovery of the Walden Inversion • In 1896, Walden showed that (-)-malic acid could be converted to (+)-malic acid by a series of chemical steps with achiral reagents Reactions of the Walden Inversions Discovery of the Walden Inversion • This established that optical rotation was directly related to chirality and that it changes with chemical alteration – Reaction of (-)-malic acid with PCl5 gives (+)chlorosuccinic acid – Further reaction with wet silver oxide gives (+)malic acid – The reaction series starting with (+)-malic acid gives (-)-malic acid Significance of the Walden Inversion • The reactions alter the array at the chirality center • The reactions involve substitution at that center • Therefore, nucleophilic substitution can invert the configuration at a chirality center • The presence of carboxyl groups in malic acid led to some dispute as to the nature of the reactions in Walden’s cycle B. Stereochemistry of Nucleophilic Substitution Kenyon and Phillips, 1929, studied the interconversion of 1-phenyl-2-propanol enantiomers to isolate step: • Only the second and fifth steps are reactions at carbon • So inversion certainly occurs in the substitution step The inversion of stereochemical configuration takes place in the second step, the nucleophilic substitution of tosylate ion by acetate ion Practice Problem: What product would you expect to obtain from a nucleophilic substitution reaction of (S)-2bromohexane with acetate ion, CH3CO2-? Assume that inversion of configuration occurs, and show the stereochemistry of both reactant and product C. Kinetics of Nucleophilic Substitution • Reaction rate – is the exact rate at which a reactant is converted into product • Kinetics – is useful for helping determine reaction mechanisms Definitions of Terms • Rate (V) - is change in concentration with time - depends on concentration(s), temperature, inherent nature of reaction (barrier on energy surface) • A rate law - describes relationship between the concentration of reactants and conversion to products • A rate constant (k) - is the proportionality factor between concentration and rate Example: for S converting to P V = d[S]/dt = k [S] • Kinetics – is the study of rates of reactions • Rates decrease as concentrations decrease but the rate constant does not • Rate units: [concentration]/time such as L/(mol x s) • The rate law – is a result of the mechanism • The order of a reaction – is sum of the exponents of the concentrations in the rate law • Second-order reaction – is a reaction in which the rate is linearly dependent on the concentration of two species Reaction rate = k x [RX] x [OH-] where [RX] = CH3Br concentration [OH-] = OH- concentration k = a constant value D. The SN2 Reaction • SN2 reaction – Substitution – Nucleophilic – Bimolecular – Bimolecular - Nu and RX take part in the step whose kinetics are measured – Second-order kinetics: rate = k x [RX] x [Nu] – Inversion of stereochemistry at the carbon atom – No intermediate/ Single step The entering Nu approaches the halide from a direction 180o away from the leaving group, resulting in an umbrella-like inversion The transition state is planar Practice Problem: What product would you expect to obtain from SN2 reaction of OH- with (R)-2-bromobutane? Show the stereochemistry of both reactant and product. Practice Problem: Assign configuration to the following substance, and draw the structure of the product that would result on nucleophilic substitution reaction with HS- (reddish-brown = Br) E. SN2 Reaction Characteristics • The effects of four variables on SN2 reactions: – Substrate: SN2 reactions are best for methyl and primary substrates – Nucleophile: Basic, negatively charged nucleophiles are more effective than neutral ones – Leaving group: Stable anions that are weak bases are good leaving groups – Solvent: Polar aprotic solvents Reactant and Transition-state Energy Levels Affect Rate • Higher reactant energy level (red curve) = faster reaction (smaller G‡). • Higher transition-state energy level (red curve) = slower reaction (larger G‡). The Substrate: Steric Effects in the SN2 Reaction • SN2 reactions are sensitive to steric effects • SN2 reactions occur only at relatively unhindered sites – Methyl halides are most reactive – Primary are next most reactive – Secondary might react – Tertiary are unreactive by this path – No reaction at C=C (vinyl halides) and aryl halides SN2 reactions are sensitive to steric effects The carbon atom in (a) bromomethane is readily accessible resulting in a fast SN2 reaction. The carbon atoms in (b) bromoethane (primary), (c) 2-bromopropane (secondary), and (d) 2-bromo-2-methylpropane (tertiary) are successively more hindered, resulting in successively slower SN2 reactions. Order of Reactivity in SN2 • The more alkyl groups connected to the reacting carbon or near it, the slower the reaction – – – – Methyl halides are most reactive Primary are next most reactive Secondary might react Tertiary are unreactive by this path • No reaction at C=C (vinyl halides) and aryl halides – This is due to steric factors The Substrate: Steric Effects in the SN2 Reaction • SN2 reactions are best for methyl and primary substrates • Steric Hindrance raises Transition State Energy, thus increasing G‡ and decreasing the reaction rate – Steric effects destabilize transition states – Severe steric effects can also destabilize ground state The Nucleophile • Neutral or negatively charged Lewis base • Reaction increases coordination at nucleophile – Neutral nucleophile acquires positive charge – Anionic nucleophile becomes neutral Relative Reactivity of Nucleophiles • It depends on substrate, solvent, and reactant concentration – More basic nucleophiles react faster (for similar structures) – Better nucleophiles are lower in a column of the periodic table – Anions are usually more reactive than neutrals Relative Reactivity of Nucleophiles • Nucleophilicity roughly parallels basicity – More basic nucleophiles react faster (for similar structures) – Nucleophilicity measures the affinity of a Lewis base for carbon atom – Basicity measures the affinity of a base for a proton Relative Reactivity of Nucleophiles • Nucleophilicity roughly parallels basicity • Nucleophilicity usually increases going down a column of the periodic table • Negatively charged Nu are usually more reactive than neutral ones Practice Problem: What product would you expect from SN2 reaction of 1-bromobutane with each of the following? a) NaI b) KOH c) H-CΞC-Li d) NH3 Practice Problem: Which substance in each of the following pairs is more reactive as a nucleophile? a) (CH3)2N- or (CH3)2NH b) (CH3)3B or (CH3)3N c) H2O or H2S The Leaving Group • A good leaving group – reduces the barrier to a reaction – stabilizes the negative charge well – is a weak base (i.e. anion derived from strong acids) • Stable anions that are weak bases are usually excellent leaving groups – They can delocalize charge • Stable anions that are weak bases are usually excellent leaving groups due to T.S formed – They distribute the negative charge over both the Nu and the leaving group – The greater the extent of charge stabilization, the lower the energy of the transition state and the more rapid the reaction Poor Leaving Groups • If a group is very basic or very small, it prevents reaction Practice Problem: Rank the following compounds in order of their expected reactivity toward SN2 reaction: CH3Br, CH3OTos, (CH3)3CCl, (CH3)2CHCl CH3Br CH3OTos (CH3)3CCl (CH3)2CHCl The Solvent • Protic solvents (with -OH or -NH groups) that can form hydrogen bonds slow SN2 reactions by associating with reactants (solvation) – Energy is required to break interactions between reactant and solvent • Polar aprotic solvents (no -NH, -OH, -SH) form weaker interactions with substrate and permit faster reaction • Protic solvents i.e. solvents that can form hydrogen bonds (-OH or -NH) slow SN2 reactions – They cluster around or solvate the reactant nucleophile lowering its ground-state energy and reactivity • Polar aprotic solvents (no NH, OH, SH) form weaker interactions with substrate and permit faster reaction – They increase the rate of SN2 reactions by raising the ground-state energy of the Nu. HMPA = hexamethylphosphoramide • Examples of polar aprotic solvents (no NH, OH, SH) include: – DMF = dimethyl formamide (CH3)2NCHO – DMSO = dimethyl sulfoxide (CH3)2SO – HMPA = hexamethylphosphoramide [(CH3)2N]3PO – acetonitrile CH3CN • Due to their high polarity, these solvents solvate metal cations rather than nucleophilic anions Practice Problem: Organic solvents such as benzene, ether, and chloroform are neither protic nor strongly polar. What effect would you expect these solvents to have on the reactivity of a nucleophile in SN2 reactions? SN2 Reaction Characteristics: Summary Substrate: SN2 reactions are best for methyl and primary substrates – Steric hindrance raises the energy of the transition state, thus increasing G‡ and decreasing the reaction rate. Nucleophile: Basic, negatively charged nucleophiles are more effective than neutral ones – More reactive nucleophiles are less stable and have a higher ground-state energy, thereby decreasing G‡ Leaving group: Stable anions that are weak bases are good leaving groups – Good leaving groups (more stable anions) lower the energy of the transition state, thus decreasing G‡ and increasing the reaction rate. Solvent: Polar aprotic solvents are the best. – Protic solvents solvate the nucleophile, thereby lowering the ground-state energy, increasing G‡ , and decreasing the reaction rate. Polar aprotic solvents surround the accompanying cation but not the nucleophilic anion, thereby raising the ground-state energy of the nucleophile, decreasing G‡, and increasig the reaction rate F. The SN1 Reaction • SN1 reaction – Substitution – Nucleophilic – Unimolecular – Unimolecular - Only RX takes part in the step whose kinetics are measured – First-order kinetics: rate = k x [RX] – Carbocation intermediate/ Two-step G. Kinetics of the SN1 Reaction • The overall rate of a reaction: – is controlled by the rate of the slowest step (i.e rate-limiting step) – depends on the concentration of the species and the rate constant of the step: Rate = k[RX] • The rate-determining step - is the highest energy transition state point on the diagram (which is not always the highest barrier) – This is the greatest difference but not necessarily the absolute highest point (the same step is rate-determining in both directions) The SN1 reaction occurs when the substrate spontaneously dissociates to a carbocation in a slow rate-limiting step, followed by a rapid reaction with nucleophile The rate-limiting step is spontaneous dissociation of the alkyl halide to give a carbocation H. Stereochemistry of the SN1 Reaction • SN1 occurs via a carbocation intermediate – A carbocation is planar, sp2-hybridized and achiral • The planar intermediate leads to loss of chirality – A free carbocation is achiral – It can react with a Nu equally well from either side • Product is racemic with a slight more inversion SN1 in Reality • Carbocation is biased to react on side opposite leaving group – This suggests that reaction occurs with carbocation loosely associated with leaving group during nucleophilic addition (Winstein) – The alternative that SN2 is also occurring is unlikely Effects of Ion Pair Formation • If leaving group remains loosely associated, then product has more inversion than retention – Associated carbocation and leaving group is an ion pair • Product is only partially racemic with more inversion than retention – The leaving group shields one side of the carbocation from the Nu Practice Problem: What product would you expect from reaction of (S)-3-chloro-3-methyloctane with acetic acid? Show the stereochemistry of both reactant and product. Practice Problem: Among the numerous examples of SN1 reactions that occur with incomplete racemization is one reported by Winstein in 1952. The optically pure tosylate of 2,2-dimethyl-1-phenyl-1-propanol ([a]D = -30.3o) was heated in acetic acid to yield the corresponding acetate ([a]D = +5.3o). If complete inversion had occurred, the optically pure acetate would have had [a]D = +53.6o. What percentage racemization and what percentage inversion occurred in this reaction? Practice Problem: Assign configuration to the following substrate, and show the stereochemistry and identity of the product you would obtain by SN1 reaction with water (reddish-brown = Br). I. Characteristics of the SN1 reaction • The effects of four variables on SN1 reactions: – Substrate: SN1 reactions are best for tertiary, allylic, and benzylic halides – Nucleophile: Neutral nucleophiles are as effective as negatively charged ones – Leaving group: Stable anions that are weak bases are good leaving groups – Solvent: Polar solvents The Substrate • The more stable the carbocation intermediate, the faster the SN1 reaction • The best substrates yield the most stable carbocations. • Delocalization of cationic charge enhances stability – Allylic and benzylic carbocations are unusually stable Allylic carbocation has 2 resonance forms Benzylic carbocation has 4 resonance foms • Delocalization of cationic charge enhances stability – Primary allyl cation is more stable than primary alkyl – Primary benzyl cation is more stable than allyl – Secondary allyl or benzyl cation is as stable as tertiary alkyl • Tertiary alkyl halide is most reactive by SN1 mechanism – Controlled by stability of carbocation Allylic and Benzylic Halides • Allylic and benzylic C-X bonds are weaker than the corresponding saturated bonds and are therefore more easily broken. – Allylic and benzylic intermediates are stabilized by delocalization of charge – Primary allylic and benzylic are also more reactive in the SN2 mechanism Practice Problem: Rank the following substances in order of their expected SN1 reactivity: CH3CH2Br H2C=CHCH(Br)CH3 H2C=CHBr CH3CH(Br)CH3 Practice Problem: 3-Bromo-1-butene and 1-bromo-2-butene undergo SN1 reaction at nearly the same rate even though one is a secondary halide and the other is primary. Explain. The Leaving Group • SN1 is critically dependent on leaving group – Reactivity: the larger halides ions are better leaving groups • In acid, OH of an alcohol is protonated and leaving group is H2O, which is still less reactive than halide • p-Toluenesulfonate (TosO-) is excellent leaving group In acid, OH of an alcohol is protonated and leaving group is H2O, which is still less reactive than halide The Nucleophile • Since nucleophilic addition occurs after formation of carbocation, reaction rate is not normally affected by nature or concentration of nucleophile Practice Problem: 1-Chloro-1,2-diphenylethane reacts with the nucleophiles fluoride ion and triethylamine at the same rate, even though one is charged and one is neutral. Explain. The Solvent • The solvent is critical in SN1 • Stabilizing carbocation also stabilizes associated transition state and controls rate Solvation of a carbocation by water • Solvation of carbocation stabilizes carbocation and increases the rate of SN1 – Solvent molecules orient around the carbocation so that the electron-rich ends of the solvent dipoles face the positive charge, thereby lowering the energy of the ion and favoring its formation Solvation of a carbocation by water Polar Solvents Promote Ionization • Polar, protic and unreactive Lewis base solvents facilitate formation of R+ – Polar solvents, such as water, methanol and DMSO are good at solvating ions – Nonpolar ether and hydrocarbon solvents are very poor at solvating ions • Solvent polarity is measured as dielectric polarization (P) – Polar solvents have high P values – Nonpolar solvents have low P • SN1 takes place much more rapidly in polar solvents than in nonpolar solvents • SN1 is favored in protic solvents – the transition-state energy leading to carbocation intermediate is lowered by solvation – Polar solvent stabilizes transition state and intermediate more than reactant and product SN1 Reaction Characteristics: Summary Substrate: SN1 reactions are best for tertiary, allylic, and benzylic halides – The best substrates yield the most stable carbocations Nucleophile: Neutral nucleophiles are as effective as negatively charged ones – The nucleophile does not affect the reaction rate, but it must be nonbasic to prevent a competitive elimination of HX. Leaving group: Stable anions that are weak bases are good leaving groups – Good leaving groups (more stable anions) lower the energy of the transition state, thus decreasing G‡ and increasing the reaction rate. Solvent: Polar solvents stabilize the carbocation intermediate by solvation, thereby increasing the reaction rate. Practice Problem: Predict whether each of the following substitution reactions is likely to be SN1 or SN2: J. Elimination Reactions of Alkyl Halides: Zaitsev’s Rule • Nu can either: – react at carbon and substitute for X or – react at a neighboring H and cause elimination • Elimination is an alternative pathway to substitution – It is opposite of addition – It generates an alkene – It can compete with substitution and decrease yield, especially for SN1 processes Zaitsev’s Rule for Elimination Reactions (1875) • Zaitsev’s Rule: In the elimination of HX from an alkyl halide, the more highly substituted alkene product predominates Mechanisms of Elimination Reactions • Ingold nomenclature: E – “elimination” • E1: X- leaves first to generate a carbocation – A base abstracts a proton from the carbocation • E2: Concerted transfer of a proton to a base and departure of leaving group Practice Problem: Ignoring double-bond stereochemistry, what products would you expect from elimination reactions of the following alkyl halides? Which product will be major in each case? Practice Problem: What alkyl halides might the following alkenes have been made from? K. The E2 Reaction • E2 reaction – Elimination – Bimolecular – Bimolecular - Base and RX take part in the step whose kinetics are measured – Second-order kinetics: rate = k x [RX] x [Base] – Periplanar geometric requirement (stereochemistry) – No intermediate/ Single step The E2 reaction takes place in a single step through a transition state in which the double bond begins to form at the same time H and X groups are leaving • E2 reaction mechanism occurs in one step without intermediates: – A proton is transferred to base as leaving group begins to depart – Transition state combines leaving of X and transfer of H – Product alkene forms stereospecifically E2 Reaction Kinetics • E2 has second-order kinetics: rate = k x [RX] x [Base] • E2 has a single, rate-limiting step: rate law has base and alkyl halide • E2 goes faster with stronger base, better leaving group Stereochemistry of E2 Reactions • E2 always occurs with periplanar geometry: – all four reacting atoms lie in the same plane • Two periplanar geometries are possible: – syn periplanar: H and X are on the same side – anti periplanar: H and X are on opposite sides Anti periplanar geometry is lower in energy because it allows the substituents to be staggered; syn periplanar requires that the substituents be eclipsed • E2 must have anti periplanar geometry: – Anti periplanar allows orbital overlap and minimizes steric interactions • E2 must have anti periplanar geometry: – Overlap of the developing orbital in the transition state requires periplanar geometry, anti arrangement Allows orbital overlap • E2’s anti periplanar geometry is somewhat similar to SN2 180o geometry: – SN2: Electron pair from Nu pushes out X on opposite side – E2: Electron pair from C-H pushes out X on opposite side • E2 is stereospecific Example: Meso-1,2-dibromo-1,2-diphenylethane with base gives (E)-1-bromo-1,2-diphenylethylene Practice Problem: What stereochemistry do you expect for the alkene obtained by E2 elimination of (1R,2R)1,2-dibromo-1,2-diphenylethane? Draw a Newman projection of the reacting conformation Practice Problem: What stereochemistry do you expect for the trisubstituted alkene obtained by E2 elimination of the following alkyl halide on treatment with KOH (Reddish-brown = Br) L. Elimination From Cyclohexanes • To undergo E2 reaction, a cyclohexane in chair conformation should have anti periplanar geometry – The hydrogen and the leaving group must be trans diaxial – This overrides Zaitsev‘s rule • Abstracted proton and leaving group should align trans-diaxial to be anti periplanar in approaching transition state – Equatorial groups are not in proper alignment • Anti periplanar requirement for E2 overrides Zaitsev‘s rule Zaitsev’s product Higher-energy chair conformation Non- Zaitsev’s product Practice Problem: Which isomer would you expect to undergo E2 elimination faster, trans-1-bromo-4-tertbutylcyclohexane or cis-1-bromo-4-tertbutylcyclohexane ? Draw each molecule in its more stable chair conformation, and explain your answer. M. The Deuterium Isotope Effect • The deuterium isotope effect can be used to determine E2 mechanism: – C-H is weaker than C-D – C-H is easier and faster to break than C-D – Substitute deuterium for hydrogen • Effect on rate is kinetic isotope effect – kH/kD = deuterium isotope effect • Rate is reduced in E2 reaction – Heavier isotope bond is slower to break – Shows C-H bond is broken in the rate-limiting step N. The E1 Reaction • E1 reaction – Elimination – Unimolecular – Unimolecular - Only RX takes part in the step whose kinetics are measured – First-order kinetics: rate = k x [RX] – No geometric requirement (stereochemistry) – Carbocation intermediate/ Two- step The E1 reaction occurs when the substrate spontaneously dissociates to a carbocation in a slow rate-limiting step, followed by loss of H+ • E1 reaction competes with SN1 mechanism in a protic solvent and with a nonbasic nucleophile: – E1 mechanism: Unimolecular dissociation of substrate to produce a carbocation intermediate in a rate-limiting step followed by loss of H+ – Best E1 substrates are also best SN1 substrates SN1 E1 E1 Reaction Kinetics • E1 has first-order kinetics: rate = k x [RX] • E1 has a rate-limiting first step Unimolecular spontaneous dissociation is the slowest step • E1 does not go any faster with stronger base Stereochemistry of E1 Reactions • E1 is not stereospecific – It does not have any geometric requirement – There is no requirement for alignment – X and H are lost in different steps • E1 product has Zaitsev’s orientation – because step that controls product is loss of proton after formation of carbocation • E1 gives Zaitsev’s product as the major product – It is the more stable product Comparing E1 and E2 • Strong base is needed for E2 but not for E1 • E2 is stereospecifc, E1 is not • E1 gives Zaitsev orientation O. Summary of Reactivity: SN1, SN2, E1, E2 • Alkyl halides undergo different reactions in competition, depending on the reacting molecule and the conditions • Based on patterns, we can predict likely outcomes Primary alkyl halides – SN2: If there is a good Nu such as RS-, I-, CN-, NH3, or Br- – E2: If there is a strong, sterically hindered base such as tert-butoxide Secondary alkyl halides SN2 and E2 occur – SN2: If there is a weakly basic Nu in a polar aprotic solvent – E2: If there is a strong base such as CH3CH2O-, OH-, or NH2SN2 E2 Secondary alkyl halides Secondary allylic and benzylic halides undergo SN1 and E1 if weakly basic Nu in protic solvents Tertiary alkyl halides – E2: – SN1: – E1: If there is a strong base such OH- or ORIf there is a protic solvent competes with SN1 E2 SN1 E1 Practice Problem: Tell whether each of the following reactions is likely to be SN1, SN2, E1, or E2: P. Substitution Reactions in Synthesis • Examples of other substitution reactions include: – Acetylide ion alkylation – Synthesis of alkyl halides from alcohols Acetylide ion alkylation • It is an SN2 reaction – Substrates: Primary alkyl halides and tosylates – Nucleophile: Acetylide ion is a strong base and a good nucleophile • E2 competes with SN2 – Substrate is secondary or tertiary substrate Synthesis of alkyl halides from alcohols Synthesis of alkyl halides from alcohols Chapter 11