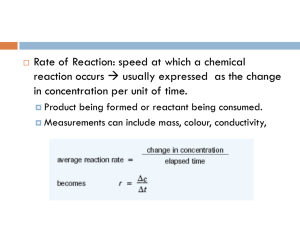
aA + bB = cC + dD
advertisement

Chapter 13 Worksheet 1 (ws13.1) Reaction Rates Reaction rate and how to measure it Consider the generalized reaction: aA + bB = cC + dD [C] Concentration (M) Concentration (M) The rate of a reaction can be measured either as the rate of disappearance of a reactant or as the rate of appearance of a product (whichever is most convenient). The graph below shows the accumulation of the product C over time. Sketch a curve that shows the loss of A over time. time (s) time (s) The average rate of the reaction over some time interval (t) is simply: Rate ΔC (unit would be M/s or mol/L-s; rates can also be expressed in moles/s) Δt Δ D We could have also expressed the rate as: or Δ A Δt Δt (the negative signs indicate a decrease in concentration.). or Δ B Δt 1. For the reaction: CS2 + 3 O2 → CO2 + 2 SO2 At a particular time in the reaction, [SO2] is changing at a rate of 0.40 M/sec. At what rate is [CO2] changing? At what rates are [CS2] and [O2] changing? [CO2]/t = [CS2]/t = (0.40 M-s-1)(1/2) = 0.20 M-s-1 (because of the 1 to 2 stoichiometry) [O2]/t = (0.40 M-s-1)(3/2) = 0.60 M-s-1 (because of the 3 to 2 stoichiometry) When reporting the rate of a reaction, you must state which reactant or product you are measuring OR do what is described below. (Note: Since the rates in this problem are “instantaneous rates” (see below) they are actually derivatives. For example: [CO2]/t = d[CO2]/dt) 1 Notice that rate of consumption of a reactant or the production of a product depends on the coefficient in the balanced equation. Therefore when reporting a rate, one must indicate the identity of the substance whose concentration was measured. For example: Δ SO2 rate = 0.40 M SO2/s or = 0.40 M/s Δt Alternatively, a single rate can be calculated that is independent of the identity of the substance. This is done as follows: Rate 1 C 1 D c t d t 1 A a t 1 B b t 2. Use this equation to calculate the rate of the chemical reaction above (independent of any particular reactant or product). Rate = (1/2)(0.40 M-s-1) = (1/1)(0.20 M-s-1) = (1/3)(0.60 M-s-1) = 0.20 M-s-1 Notice that this is the rate that one would measure for reactants or products that have a coefficient of one in the balanced equation. The instantaneous rate (the rate at any given time, t, is the slope of the concentration vs. time curve at time t (the first derivative, d[C]/dt, evaluated at time t). 3. Over time, what happens to the instantaneous rate of the reaction above? Why? (There are two reasons.) The rate (of the forward reaction) decreases (decreasing slope). Reasons: 1. Reactants are consumed so the frequency of collisions between reactants decreases 2. As soon as products begin to accumulate, the reverse reaction occurs. 4. Eventually, the reaction appears to stop? Why? (There are two possible explanations.) 1. If the reaction goes to completion then the limiting reactant is completely consumed. 2. If the reaction does not go to completion (which is usually the case), then the reaction reaches equilibrium. This occurs because over time the rate of the forward reaction decreases and the rate of the reverse reaction increases. Equilibrium is reached when the rates of the opposing reactions are equal. In this case the measured rate at each time point is actually the net rate = rate of forward reaction – rate of the reverse reaction. 2 Usually one is only interested in the rate of the forward reaction rather than the net rate (net rate = ratef – rater). There are two ways to avoid the complications that arise due to the occurrence of the reverse reaction: 1. Measure the rate early in the reaction so that there is very little product. In this way the reverse reaction is negligible. “Early” usually means the point in the reaction in which <10% of the reactants have been consumed. This is called the “initial rate” (V0). 2. Remove one or more of the products as they are made so that the reverse reaction can’t occur. (3. In some cases the reverse reaction is naturally so slow as to be insignificant. In such cases the measured rate is always the rate of the forward reaction. Such reactions go to completion.) 3